Use of biotinylated plasmid DNA as a surrogate for HSV DNA to identify proteins that repress or activate viral gene expression
- PMID: 23223531
- PMCID: PMC3529035
- DOI: 10.1073/pnas.1218783109
Use of biotinylated plasmid DNA as a surrogate for HSV DNA to identify proteins that repress or activate viral gene expression
Abstract
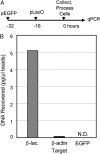
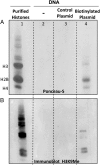
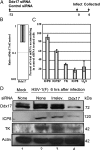
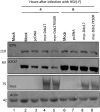
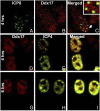
ICP0, a key herpes simplex virus regulatory protein, functions first in the nucleus and then in the cytoplasm. The duration of its nuclear sojourn in cells transfected with DNA and then infected is related to the quantity of transfected DNA. Furthermore, ICP0 transactivates both viral genes and genes encoded by the transfected DNA. The data support the hypothesis that ICP0 is retained in the nucleus until it completes the replacement of repressive chromatin with effector proteins that enable transcription of both DNA templates.To identify the effector proteins, we transfected cells with biotinylated DNA encoding a nonviral gene and then infected the cells with wild-type virus. Proteins bound to transfected biotinylated plasmid recovered from mock-treated and infected cells were identified using mass spectrometry followed by appropriate database search. The transfected DNA from mock-infected cells yielded proteins associated with repression, whereas DNA recovered from infected cells included proteins known to enable transcription and proteins that have not been previously associated with that role. To test the hypothesis that the proteins hitherto not known to associate with viral gene expression are nevertheless essential, we tested the role of the DEAD-box helicase Ddx17. We report that Ddx17 plays a critical role in the expression of early and late viral genes. Thus, biotinylated DNA recovered from transfected infected cells can function as a surrogate for viral DNA and is a rich source of proteins that play a role in viral gene expression but which have not been previously identified in that role.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Roizman B, Knipe DM, Whitley RJ. In: Fields’ Virology, Herpes simplex viruses. 5th Ed. Knipe DM, et al., editors. New York: Lippincott-Williams and Wilkins; 2007. pp. 2501–2601.
-
- Roizman B, Zhou G, Du T. Checkpoints in productive and latent infections with herpes simplex virus 1: Conceptualization of the issues. J Neurovirol. 2011;17(6):512–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

