RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release
- PMID: 23223532
- PMCID: PMC3529066
- DOI: 10.1073/pnas.1211844110
RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release
Abstract
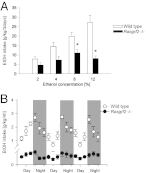
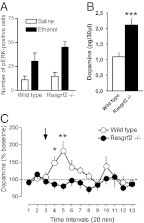
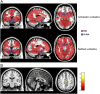
The firing of mesolimbic dopamine neurons is important for drug-induced reinforcement, although underlying genetic factors remain poorly understood. In a recent genome-wide association metaanalysis of alcohol intake, we identified a suggestive association of SNP rs26907 in the ras-specific guanine-nucleotide releasing factor 2 (RASGRF2) gene, encoding a protein that mediates Ca(2+)-dependent activation of the ERK pathway. We performed functional characterization of this gene in relation to alcohol-related phenotypes and mesolimbic dopamine function in both mice and adolescent humans. Ethanol intake and preference were decreased in Rasgrf2(-/-) mice relative to WT controls. Accordingly, ethanol-induced dopamine release in the ventral striatum was blunted in Rasgrf2(-/-) mice. Recording of dopamine neurons in the ventral tegmental area revealed reduced excitability in the absence of Ras-GRF2, likely because of lack of inhibition of the I(A) potassium current by ERK. This deficit provided an explanation for the altered dopamine release, presumably linked to impaired activation of dopamine neurons firing. Functional neuroimaging analysis of a monetary incentive-delay task in 663 adolescent boys revealed significant association of ventral striatal activity during reward anticipation with a RASGRF2 haplotype containing rs26907, the SNP associated with alcohol intake in our previous metaanalysis. This finding suggests a link between the RASGRF2 haplotype and reward sensitivity, a known risk factor for alcohol and drug addiction. Indeed, follow-up of these same boys at age 16 y revealed an association between this haplotype and number of drinking episodes. Together, these combined animal and human data indicate a role for RASGRF2 in the regulation of mesolimbic dopamine neuron activity, reward response, and alcohol use and abuse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22(11):521–527. - PubMed
-
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–69. - PubMed
-
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11(6):557–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

