Thioredoxin and thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism
- PMID: 23223577
- PMCID: PMC3561554
- DOI: 10.1074/jbc.M112.418046
Thioredoxin and thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism
Abstract
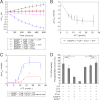
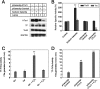
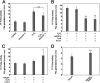
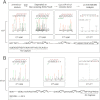
Abnormally enhanced tissue factor (TF) activity is related to increased thrombosis risk in which oxidative stress plays a critical role. Human cytosolic thioredoxin (hTrx1) and thioredoxin reductase (TrxR), also secreted into circulation, have the power to protect against oxidative stress. However, the relationship between hTrx1/TrxR and TF remains unknown. Here we show reversible association of hTrx1 with TF in human serum and plasma samples. The association is dependent on hTrx1-Cys-73 that bridges TF-Cys-209 via a disulfide bond. hTrx1-Cys-73 is absolutely required for hTrx1 to interfere with FVIIa binding to purified and cell-surface TF, consequently suppressing TF-dependent procoagulant activity and proteinase-activated receptor-2 activation. Moreover, hTrx1/TrxR plays an important role in sensing the alterations of NADPH/NADP(+) states and transducing this redox-sensitive signal into changes in TF activity. With NADPH, hTrx1/TrxR readily facilitates the reduction of TF, causing a decrease in TF activity, whereas with NADP(+), hTrx1/TrxR promotes the oxidation of TF, leading to an increase in TF activity. By comparison, TF is more likely to favor the reduction by hTrx1-TrxR-NADPH. This reversible reduction-oxidation reaction occurs in the TF extracellular domain that contains partially opened Cys-49/-57 and Cys-186/-209 disulfide bonds. The cell-surface TF procoagulant activity is significantly increased after hTrx1-knockdown. The response of cell-surface TF procoagulant activity to H(2)O(2) is efficiently suppressed through elevating cellular TrxR activity via selenium supplementation. Our data provide a novel mechanism for redox regulation of TF activity. By modifying Cys residues or regulating Cys redox states in TF extracellular domain, hTrx1/TrxR function as a safeguard against inappropriate TF activity.
Figures



References
-
- Cimmino G., Golino P., Badimon J. J. (2011) Pathophysiological role of blood-borne tissue factor. Should the old paradigm be revisited? Intern. Emerg. Med. 6, 29–34 - PubMed
-
- Belting M., Ahamed J., Ruf W. (2005) Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler. Thromb. Vasc. Biol. 25, 1545–1550 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

