Functional modeling in zebrafish demonstrates that the atrial-fibrillation-associated gene GREM2 regulates cardiac laterality, cardiomyocyte differentiation and atrial rhythm
- PMID: 23223679
- PMCID: PMC3597016
- DOI: 10.1242/dmm.010488
Functional modeling in zebrafish demonstrates that the atrial-fibrillation-associated gene GREM2 regulates cardiac laterality, cardiomyocyte differentiation and atrial rhythm
Abstract
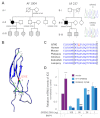
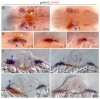
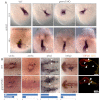
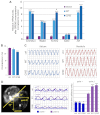
Atrial fibrillation (AF) is the most common cardiac arrhythmia and carries a significant risk of stroke and heart failure. The molecular etiologies of AF are poorly understood, leaving patients with limited therapeutic options. AF has been recognized as an inherited disease in almost 30% of patient cases. However, few genetic loci have been identified and the mechanisms linking genetic variants to AF susceptibility remain unclear. By sequencing 193 probands with lone AF, we identified a Q76E variant within the coding sequence of the bone morphogenetic protein (BMP) antagonist gremlin-2 (GREM2) that increases its inhibitory activity. Functional modeling in zebrafish revealed that, through regulation of BMP signaling, GREM2 is required for cardiac laterality and atrial differentiation during embryonic development. GREM2 overactivity results in slower cardiac contraction rates in zebrafish, and induction of previously identified AF candidate genes encoding connexin-40, sarcolipin and atrial natriuretic peptide in differentiated mouse embryonic stem cells. By live heart imaging in zebrafish overexpressing wild-type or variant GREM2, we found abnormal contraction velocity specifically in atrial cardiomyocytes. These results implicate, for the first time, regulators of BMP signaling in human AF, providing mechanistic insights into the pathogenesis of the disease and identifying potential new therapeutic targets.
Figures


References
-
- Antzelevitch C., Pollevick G. D., Cordeiro J. M., Casis O., Sanguinetti M. C., Aizawa Y., Guerchicoff A., Pfeiffer R., Oliva A., Wollnik B., et al. (2007). Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 115, 442-449 - PMC - PubMed
-
- Avsian-Kretchmer O., Hsueh A. J. (2004). Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 18, 1-12 - PubMed
-
- Bisgrove B. W., Essner J. J., Yost H. J. (1999). Regulation of midline development by antagonism of lefty and nodal signaling. Development 126, 3253-3262 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL009221/HL/NHLBI NIH HHS/United States
- HL09221/HL/NHLBI NIH HHS/United States
- HL100398/HL/NHLBI NIH HHS/United States
- HL083958/HL/NHLBI NIH HHS/United States
- R01 HL083958/HL/NHLBI NIH HHS/United States
- R01 HL092217/HL/NHLBI NIH HHS/United States
- HL65962/HL/NHLBI NIH HHS/United States
- DE018477/DE/NIDCR NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- U01 HL100398/HL/NHLBI NIH HHS/United States
- U01 HL065962/HL/NHLBI NIH HHS/United States
- R01 DE018477/DE/NIDCR NIH HHS/United States
- T32 GM008554/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

