Inactivation of enveloped virus by laser-driven protein aggregation
- PMID: 23224114
- PMCID: PMC3518210
- DOI: 10.1117/1.JBO.17.12.128002
Inactivation of enveloped virus by laser-driven protein aggregation
Abstract
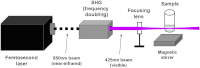
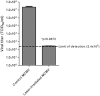
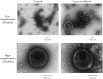
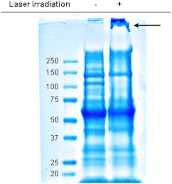
Ultrafast lasers in the visible and near-infrared range have emerged as a potential new method for pathogen reduction of blood products and pharmaceuticals. However, the mechanism of enveloped virus inactivation by this method is unknown. We report the inactivation as well as the molecular and structural effects caused by visible (425 nm) femtosecond laser irradiation on murine cytomegalovirus (MCMV), an enveloped, double-stranded DNA virus. Our results show that laser irradiation (1) caused a 5-log reduction in MCMV titer, (2) did not cause significant changes to the global structure of MCMV virions including membrane and capsid, as assessed by electron microscopy, (3) produced no evidence of double-strand breaks or crosslinking in MCMV genomic DNA, and (4) caused selective aggregation of viral capsid and tegument proteins. We propose a model in which ultrafast laser irradiation induces partial unfolding of viral proteins by disrupting hydrogen bonds and/or hydrophobic interactions, leading to aggregation of closely associated viral proteins and inactivation of the virus. These results provide new insight into the inactivation of enveloped viruses by visible femtosecond lasers at the molecular level, and help pave the way for the development of a new ultrafast laser technology for pathogen reduction.
Figures

Similar articles
-
Pathogen reduction in human plasma using an ultrashort pulsed laser.PLoS One. 2014 Nov 5;9(11):e111673. doi: 10.1371/journal.pone.0111673. eCollection 2014. PLoS One. 2014. PMID: 25372037 Free PMC article.
-
Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser.Virol J. 2014 Feb 5;11:20. doi: 10.1186/1743-422X-11-20. Virol J. 2014. PMID: 24495489 Free PMC article.
-
Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus.Antiviral Res. 2014 Oct;110:70-6. doi: 10.1016/j.antiviral.2014.07.012. Epub 2014 Jul 30. Antiviral Res. 2014. PMID: 25086212 Free PMC article.
-
Comparative study on the inactivation of MS2 and M13 bacteriophages using energetic femtosecond lasers.J Biophotonics. 2020 Oct;13(10):e202000109. doi: 10.1002/jbio.202000109. Epub 2020 Aug 10. J Biophotonics. 2020. PMID: 32701195 Review.
-
Arginine-enveloped virus inactivation and potential mechanisms.Biotechnol Prog. 2020 Mar;36(2):e2931. doi: 10.1002/btpr.2931. Epub 2019 Nov 11. Biotechnol Prog. 2020. PMID: 31622532 Review.
Cited by
-
Oxygen-dependent laser inactivation of murine norovirus using visible light lasers.Virol J. 2018 Jul 31;15(1):117. doi: 10.1186/s12985-018-1019-2. Virol J. 2018. PMID: 30064439 Free PMC article.
-
Pathogen reduction in human plasma using an ultrashort pulsed laser.PLoS One. 2014 Nov 5;9(11):e111673. doi: 10.1371/journal.pone.0111673. eCollection 2014. PLoS One. 2014. PMID: 25372037 Free PMC article.
-
Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser.Virol J. 2014 Feb 5;11:20. doi: 10.1186/1743-422X-11-20. Virol J. 2014. PMID: 24495489 Free PMC article.
-
Light-based technologies for management of COVID-19 pandemic crisis.J Photochem Photobiol B. 2020 Nov;212:111999. doi: 10.1016/j.jphotobiol.2020.111999. Epub 2020 Aug 19. J Photochem Photobiol B. 2020. PMID: 32855026 Free PMC article. Review.
-
Can biowarfare agents be defeated with light?Virulence. 2013 Nov 15;4(8):796-825. doi: 10.4161/viru.26475. Epub 2013 Sep 25. Virulence. 2013. PMID: 24067444 Free PMC article. Review.
References
-
- Horowitz B., et al. , “Solvent/detergent-treated plasma: a virus-inactivated substitute for fresh frozen plasma,” Blood 79(3), 826–831 (1992).BLOOAW - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

