C. elegans RNA-dependent RNA polymerases rrf-1 and ego-1 silence Drosophila transgenes by differing mechanisms
- PMID: 23224429
- PMCID: PMC11113355
- DOI: 10.1007/s00018-012-1218-8
C. elegans RNA-dependent RNA polymerases rrf-1 and ego-1 silence Drosophila transgenes by differing mechanisms
Abstract
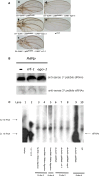
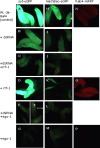
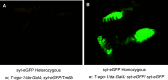
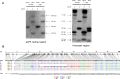
Drosophila possesses the core gene silencing machinery but, like all insects, lacks the canonical RNA-dependent RNA polymerases (RdRps) that in C. elegans either trigger or enhance two major small RNA-dependent gene silencing pathways. Introduction of two different nematode RdRps into Drosophila showed them to be functional, resulting in differing silencing activities. While RRF-1 enhanced transitive dsRNA-dependent silencing, EGO-1 triggered dsRNA-independent silencing, specifically of transgenes. The strain w; da-Gal4; UAST-ego-1, constitutively expressing ego-1, is capable of silencing transgene including dsRNA hairpin upon a single cross, which created a powerful tool for research in Drosophila. In C. elegans, EGO-1 is involved in transcriptional gene silencing (TGS) of chromosome regions that are unpaired during meiosis. There was no opportunity for meiotic interactions involving EGO-1 in Drosophila that would explain the observed transgene silencing. Transgene DNA is, however, unpaired during the pairing of chromosomes in embryonic mitosis that is an unusual characteristic of Diptera, suggesting that in Drosophila, EGO-1 triggers transcriptional silencing of unpaired DNA during embryonic mitosis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

