A proposal for an additional clinical trial outcome measure assessing preventive effect as delay of events
- PMID: 23224516
- PMCID: PMC3539066
- DOI: 10.1007/s10654-012-9752-0
A proposal for an additional clinical trial outcome measure assessing preventive effect as delay of events
Abstract
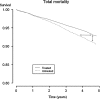
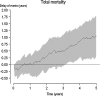
Many effect measures used in clinical trials are problematic because they are differentially understood by patients and physicians. The emergence of novel methods such as accelerated failure-time models and quantile regression has shifted the focus of effect measurement from probability measures to time-to-event measures. Such modeling techniques are rapidly evolving, but matching non-parametric descriptive measures are lacking. We propose such a measure, the delay of events, demonstrating treatment effect as a gain in event-free time. We believe this measure to be of value for shared clinical decision-making. The rationale behind the measure is given, and it is conceptually explained using the Kaplan-Meier estimate and the quantile regression framework. A formula for calculation of the delay of events is given. Hypothetical and empirical examples are used to demonstrate the measure. The measure is discussed in relation to other measures highlighting the time effects of preventive treatments. There is a need to further investigate the properties of the measure as well as its role in clinical decision-making.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources

