AMP-activated protein kinase (AMPK)α2 plays a role in determining the cellular fate of glucose in insulin-resistant mouse skeletal muscle
- PMID: 23224579
- PMCID: PMC4075509
- DOI: 10.1007/s00125-012-2787-7
AMP-activated protein kinase (AMPK)α2 plays a role in determining the cellular fate of glucose in insulin-resistant mouse skeletal muscle
Abstract
Aims/hypothesis: We determined whether: (1) an acute lipid infusion impairs skeletal muscle AMP-activated protein kinase (AMPK)α2 activity, increases inducible nitric oxide synthase (iNOS) and causes peripheral insulin resistance in conscious, unstressed, lean mice; and (2) restoration of AMPKα2 activity during the lipid infusion attenuates the increase in iNOS and reverses the defect in insulin sensitivity in vivo.
Methods: Chow-fed, 18-week-old C57BL/6J male mice were surgically catheterised. After 5 days they received: (1) a 5 h infusion of 5 ml kg(-1) h(-1) Intralipid + 6 U/h heparin (Lipid treatment) or saline (Control); (2) Lipid treatment or Control, followed by a 2 h hyperinsulinaemic-euglycaemic clamp (insulin clamp; 4 mU kg(-1) min(-1)); and (3) infusion of the AMPK activator, 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR) (1 mg kg(-1) min(-1)), or saline during Lipid treatment, followed by a 2 h insulin clamp. In a separate protocol, mice producing a muscle-specific kinase-dead AMPKα2 subunit (α2-KD) underwent an insulin clamp to determine the role of AMPKα2 in insulin-mediated muscle glucose metabolism.
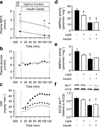
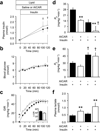
Results: Lipid treatment decreased AMPKα2 activity, increased iNOS abundance/activation and reduced whole-body insulin sensitivity in vivo. AICAR increased AMPKα2 activity twofold; this did not suppress iNOS or improve whole-body or tissue-specific rates of glucose uptake during Lipid treatment. AICAR caused a marked increase in insulin-mediated glycogen synthesis in skeletal muscle. Consistent with this latter result, lean α2-KD mice exhibited impaired insulin-stimulated glycogen synthesis even though muscle glucose uptake was not affected.
Conclusions/interpretation: Acute induction of insulin resistance via lipid infusion in healthy mice impairs AMPKα2, increases iNOS and causes insulin resistance in vivo. However, these changes do not appear to be interrelated. Rather, a functionally active AMPKα2 subunit is required for insulin-stimulated muscle glycogen synthesis.
Figures




References
-
- de Lange P, Moreno M, Silvestri E, Lombardi A, Goglia F, Lanni A. Fuel economy in food-deprived skeletal muscle: signaling pathways and regulatory mechanisms. FASEB J. 2007;21:3431–3441. - PubMed
-
- Seufert J. A fixed-dose combination of pioglitazone and metformin: A promising alternative in metabolic control. Curr Med Res Opin. 2006;22(Suppl 2):S39–S48. - PubMed
-
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. - PubMed
-
- Iglesias MA, Ye JM, Frangioudakis G, et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

