Cell signaling pathways in vertebrate lens regeneration
- PMID: 23224710
- PMCID: PMC4304700
- DOI: 10.1007/82_2012_289
Cell signaling pathways in vertebrate lens regeneration
Abstract
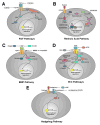
Certain vertebrates are capable of regenerating parts of the eye, including the lens. Depending on the species, two principal forms of in vivo lens regeneration have been described wherein the new lens arises from either the pigmented epithelium of the dorsal iris or the cornea epithelium. These forms of lens regeneration are triggered by retinal factors present in the eye. Studies have begun to illuminate the nature of the signals that support lens regeneration. This review describes evidence for the involvement of specific signaling pathways in lens regeneration, including the FGF, retinoic acid, TGF-beta, Wnt, and Hedgehog pathways.
Figures

Similar articles
-
Lens regeneration from the cornea requires suppression of Wnt/β-catenin signaling.Exp Eye Res. 2016 Apr;145:206-215. doi: 10.1016/j.exer.2016.01.003. Epub 2016 Jan 8. Exp Eye Res. 2016. PMID: 26778749 Free PMC article.
-
Retinoic acid regulation by CYP26 in vertebrate lens regeneration.Dev Biol. 2014 Feb 15;386(2):291-301. doi: 10.1016/j.ydbio.2013.12.036. Epub 2013 Dec 30. Dev Biol. 2014. PMID: 24384390 Free PMC article.
-
The cellular and molecular bases of vertebrate lens regeneration.Int Rev Cytol. 2003;228:195-265. doi: 10.1016/s0074-7696(03)28005-0. Int Rev Cytol. 2003. PMID: 14667045 Review.
-
Conservation of fibroblast growth factor function in lens regeneration.Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13701-6. doi: 10.1073/pnas.94.25.13701. Proc Natl Acad Sci U S A. 1997. PMID: 9391089 Free PMC article.
-
[Cell sources, regulatory factors and gene expression in the regeneration of the crystalline lens and retina in vertebrate animals].Izv Akad Nauk Ser Biol. 1996 May-Jun;(3):298-318. Izv Akad Nauk Ser Biol. 1996. PMID: 8755029 Review. Russian.
Cited by
-
Lens regeneration from the cornea requires suppression of Wnt/β-catenin signaling.Exp Eye Res. 2016 Apr;145:206-215. doi: 10.1016/j.exer.2016.01.003. Epub 2016 Jan 8. Exp Eye Res. 2016. PMID: 26778749 Free PMC article.
-
Lens regeneration: scientific discoveries and clinical possibilities.Mol Biol Rep. 2021 May;48(5):4911-4923. doi: 10.1007/s11033-021-06489-5. Epub 2021 Jun 18. Mol Biol Rep. 2021. PMID: 34143397 Review.
-
Lens regeneration in humans: using regenerative potential for tissue repairing.Ann Transl Med. 2020 Nov;8(22):1544. doi: 10.21037/atm-2019-rcs-03. Ann Transl Med. 2020. PMID: 33313289 Free PMC article. Review.
-
Diverse Evolutionary Origins and Mechanisms of Lens Regeneration.Mol Biol Evol. 2018 Jul 1;35(7):1563-1575. doi: 10.1093/molbev/msy045. Mol Biol Evol. 2018. PMID: 29579253 Free PMC article. Review.
-
Go ahead, grow a head! A planarian's guide to anterior regeneration.Regeneration (Oxf). 2016 Jun 24;3(3):139-55. doi: 10.1002/reg2.56. eCollection 2016 Jun. Regeneration (Oxf). 2016. PMID: 27606065 Free PMC article. Review.
References
-
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–295. - PubMed
-
- Arresta E, Bernardini S, Gargioli C, Filoni S, Cannata SM. Lens-forming competence in the epidermis of Xenopus laevis during development. J Exp Zool. 2005;303A:1–12. - PubMed
-
- Blobe G, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor β (TGF-β) signaling. Functional modulation of type III TGF-b receptor expression through interaction with the PDZ domain protein. GIPC J Biol Chem. 2001;276:39608–39617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

