Cross talk between plasma membrane Na(+)/Ca (2+) exchanger-1 and TRPC/Orai-containing channels: key players in arterial hypertension
- PMID: 23224895
- PMCID: PMC3857394
- DOI: 10.1007/978-1-4614-4756-6_31
Cross talk between plasma membrane Na(+)/Ca (2+) exchanger-1 and TRPC/Orai-containing channels: key players in arterial hypertension
Abstract
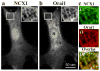
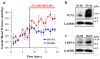
Arterial smooth muscle (ASM) Na(+)/Ca(2+) exchanger type 1 (NCX1) and TRPC/Orai-containing receptor/store-operated cation channels (ROC/SOC) are clustered with α2 Na(+) pumps in plasma membrane microdomains adjacent to the underlying junctional sarcoplasmic reticulum. This arrangement enables these transport proteins to function as integrated units to help regulate local Na(+) metabolism, Ca(2+) signaling, and arterial tone. They thus influence vascular resistance and blood pressure (BP). For instance, upregulation of NCX1 and TRPC6 has been implicated in the pathogenesis of high BP in several models of essential hypertension. The models include ouabain-induced hypertensive rats, Milan hypertensive rats, and Dahl salt-sensitive hypertensive rats, all of which exhibit elevated plasma ouabain levels. We suggest that these molecular mechanisms are key contributors to the increased vascular resistance ("whole body autoregulation") that elevates BP in essential hypertension. Enhanced expression and function of ASM NCX1 and TRPC/Orai1-containing channels in hypertension implies that these proteins are potential targets for pharmacological intervention.
Figures
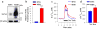
Similar articles
-
Upregulation of Na+/Ca2+ exchanger and TRPC6 contributes to abnormal Ca2+ homeostasis in arterial smooth muscle cells from Milan hypertensive rats.Am J Physiol Heart Circ Physiol. 2010 Sep;299(3):H624-33. doi: 10.1152/ajpheart.00356.2010. Epub 2010 Jul 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20622104 Free PMC article.
-
Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats.Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H263-74. doi: 10.1152/ajpheart.00784.2009. Epub 2009 Nov 6. Am J Physiol Heart Circ Physiol. 2010. PMID: 19897708 Free PMC article.
-
Nanomolar ouabain increases NCX1 expression and enhances Ca2+ signaling in human arterial myocytes: a mechanism that links salt to increased vascular resistance?Am J Physiol Heart Circ Physiol. 2012 Oct 1;303(7):H784-94. doi: 10.1152/ajpheart.00399.2012. Epub 2012 Jul 27. Am J Physiol Heart Circ Physiol. 2012. PMID: 22842068 Free PMC article.
-
New insights into the contribution of arterial NCX to the regulation of myogenic tone and blood pressure.Adv Exp Med Biol. 2013;961:329-43. doi: 10.1007/978-1-4614-4756-6_28. Adv Exp Med Biol. 2013. PMID: 23224892 Review.
-
Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC proteins.Biochim Biophys Acta. 2010 Dec;1802(12):1219-29. doi: 10.1016/j.bbadis.2010.02.011. Epub 2010 Mar 6. Biochim Biophys Acta. 2010. PMID: 20211726 Free PMC article. Review.
Cited by
-
The pump, the exchanger, and the holy spirit: origins and 40-year evolution of ideas about the ouabain-Na+ pump endocrine system.Am J Physiol Cell Physiol. 2018 Jan 1;314(1):C3-C26. doi: 10.1152/ajpcell.00196.2017. Epub 2017 Nov 7. Am J Physiol Cell Physiol. 2018. PMID: 28971835 Free PMC article. Review.
-
Endogenous Ouabain: Recent Advances and Controversies.Hypertension. 2016 Sep;68(3):526-32. doi: 10.1161/HYPERTENSIONAHA.116.06599. Epub 2016 Jul 25. Hypertension. 2016. PMID: 27456525 Free PMC article. Review.
-
Sensational site: the sodium pump ouabain-binding site and its ligands.Am J Physiol Cell Physiol. 2024 Apr 1;326(4):C1120-C1177. doi: 10.1152/ajpcell.00273.2023. Epub 2024 Jan 15. Am J Physiol Cell Physiol. 2024. PMID: 38223926 Free PMC article. Review.
-
Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension.J Cereb Blood Flow Metab. 2013 Nov;33(11):1732-42. doi: 10.1038/jcbfm.2013.143. Epub 2013 Aug 14. J Cereb Blood Flow Metab. 2013. PMID: 23942363 Free PMC article.
-
Update on angiotensin II: new endocrine connections between the brain, adrenal glands and the cardiovascular system.Endocr Connect. 2017 Oct;6(7):R131-R145. doi: 10.1530/EC-17-0161. Epub 2017 Aug 30. Endocr Connect. 2017. PMID: 28855243 Free PMC article. Review.
References
-
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–173. - PubMed
-
- Bae YM, Kim A, Lee YJ, Lim W, Noh YH, Kim EJ, Kim J, Kim TK, Park SW, Kim B, Cho SI, Kim DK, Ho WK. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2007;25:809–817. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

