Relevance of PepT1 in the intestinal permeability and oral absorption of cefadroxil
- PMID: 23224978
- PMCID: PMC3596500
- DOI: 10.1007/s11095-012-0937-8
Relevance of PepT1 in the intestinal permeability and oral absorption of cefadroxil
Abstract
Purpose: To determine the contribution of intestinal PepT1 on the permeability and oral absorption of the β-lactam antibiotic drug cefadroxil.
Methods: The effective permeability (P eff ) of cefadroxil was evaluated in wild-type and PepT1 knockout mice following in situ single-pass intestinal perfusions. The plasma concentration-time profiles of cefadroxil were also examined after oral gavage.
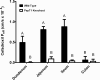
Results: The P eff (cm/s) of cefadroxil in wild-type mice was 0.49 × 10(-4) in duodenum, 0.80 × 10(-4) in jejunum, 0.88 × 10(-4) in ileum and 0.064 × 10(-4) in colon. The P eff (cm/s) in PepT1 knockout mice was significantly reduced in small intestine, but not in colon, as shown by values of 0.003 × 10(-4), 0.090 × 10(-4), 0.042 × 10(-4) and 0.032 × 10(-4), respectively. Jejunal uptake of cefadroxil was saturable (Km = 2-4 mM) and significantly attenuated by the sodium-proton exchange inhibitor 5-(N,N-dimethyl)amiloride. Jejunal permeability of cefadroxil was not affected by L-histidine, glycine, cephalothin, p-aminohippurate or N-methylnicotinamide. In contrast, cefadroxil permeability was significantly reduced by glycylproline, glycylsarcosine, or cephalexin. Finally, PepT1 ablation resulted in 23-fold reductions in peak plasma concentrations and 14-fold reductions in systemic exposure of cefadroxil after oral dosing.
Conclusions: The findings are definitive in demonstrating that PepT1 is the major transporter responsible for the small intestinal permeability of cefadroxil as well as its enhanced oral drug performance.
Figures








References
-
- Daniel H, Spanier B, Kottra G, Weitz D. From bacteria to man: archaic proton-dependent peptide transporters at work. Physiol. 2006;21:93–102. - PubMed
-
- Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2008;38:1022–1042. - PubMed
-
- Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–585. - PubMed
-
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

