Visible and near infrared fluorescence spectral flow cytometry
- PMID: 23225549
- PMCID: PMC3594514
- DOI: 10.1002/cyto.a.22241
Visible and near infrared fluorescence spectral flow cytometry
Abstract
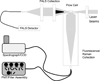
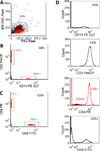
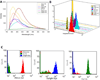
There is a long standing interest in measuring complete emission spectra from individual cells in flow cytometry. We have developed flow cytometry instruments and analysis approaches to enable this to be done routinely and robustly. Our spectral flow cytometers use a holographic grating to disperse light from single cells onto a CCD for high speed, wavelength-resolved detection. Customized software allows the single cell spectral data to be displayed and analyzed to produce new spectra-derived parameters. We show that familiar reference and calibration beads can be employed to quantitatively assess instrument performance. We use microspheres stained with six different quantum dots to compare a virtual bandpass filter approach with classic least squares (CLS) spectral unmixing, and then use antibody capture beads and CLS unmixing to demonstrate immunophenotyping of peripheral blood mononuclear cells using spectral flow cytometry. Finally, we characterize and evaluate several near infrared (NIR) emitting fluorophores for use in spectral flow cytometry. Spectral flow cytometry offers a number of attractive features for single cell analysis, including a simplified optical path, high spectral resolution, and streamlined approaches to quantitative multiparameter measurements. The availability of robust instrumentation, software, and analysis approaches will facilitate the development of spectral flow cytometry applications.
Copyright © 2012 International Society for Advancement of Cytometry.
Figures





References
-
- Asbury CL, Esposito R, Farmer C, van den Engh G. Fluorescence spectra of DNA dyes measured in a flow cytometer. Cytometry. 1996;24:234–242. - PubMed
-
- Buican TN. Real-time Fourier transform spectrometry for fluorescence imaging and flow cytometry. 1990:126.
-
- Dubelaar GBJ, Gerritzen PL, Beeker AER, Jonker RR, Tangen K. Design and first results of CytoBuoy: A wireless flow cytometer for in situ analysis of marine and fresh waters. Cytometry. 1999;37:247–254. - PubMed
-
- Fuller RR, Sweedler JV. Characterizing submicron vesicles with wavelength?resolved fluorescence in flow cytometry. Cytometry. 1996;25:144–155. - PubMed
-
- Gauci M, Vesey G, Narai J, Veal D, Williams K, Piper J. Observation of single?cell fluorescence spectra in laser flow cytometry. Cytometry. 1996;25:388–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

