A fluorescence-based assay for p38α recruitment site binders: identification of rooperol as a novel p38α kinase inhibitor
- PMID: 23225637
- PMCID: PMC3775607
- DOI: 10.1002/cbic.201200529
A fluorescence-based assay for p38α recruitment site binders: identification of rooperol as a novel p38α kinase inhibitor
Abstract
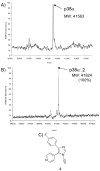
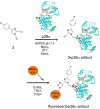
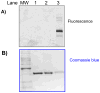
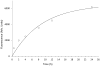
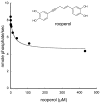
A new p38α inhibitor: Using a D-recruitment site (DRS) probe for p38α which exploits covalent interaction with Cys119 and alkyne-azide "click" chemistry to identify small molecules that recognize the p38α DRS, the anti-inflammatory natural product rooperol was identified as a novel p38α inhibitor.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



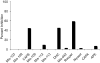
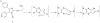
References
-
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. Chem Rev. 2001;101:2449–2476. - PubMed
-
- Johnson GL, Lapadat R. Science. 2002;298:1911–1912. - PubMed
-
- Dhillon AS, Hagan S, Rath O, Kolch W. Oncogene. 2007;26:3279–3290. - PubMed
-
- Regan J, Breitfelder S, Cirillo P, Gilmore T, Graham AG, Hickey E, Klaus B, Madwed J, Moriak M, Moss N, Pargellis C, Pav S, Proto A, Swinamer A, Tong L, Torcellini C. J Med Chem. 2002;45:2994–3008. - PubMed
-
- Wrobleski ST, Doweyko AM. Curr Top Med Chem. 2005;5:1005–1016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

