Effect of mitomycin on normal dermal fibroblast and HaCat cell: an in vitro study
- PMID: 23225855
- PMCID: PMC3520454
- DOI: 10.1631/jzus.B1200055
Effect of mitomycin on normal dermal fibroblast and HaCat cell: an in vitro study
Abstract
Objective: To evaluate the effects of mitomycin on the growth of human dermal fibroblast and immortalized human keratinocyte line (HaCat cell), particularly the effect of mitomycin on intracellular messenger RNA (mRNA) synthesis of collagen and growth factors of fibroblast.
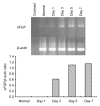
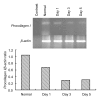
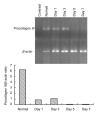
Methods: The normal dermal fibroblast and HaCat cell were cultured in vitro. Cell cultures were exposed to 0.4 and 0.04 mg/ml of mitomycin solution, and serum-free culture medium was used as control. The cellular morphology change, growth characteristics, cell proliferation, and apoptosis were observed at different intervals. For the fibroblasts, the mRNA expression changes of transforming growth factor (TGF)-β1, basic fibroblast growth factor (bFGF), procollagen I, and III were detected by reverse transcription polymerase chain reaction (RT-PCR).
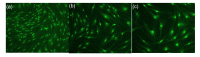
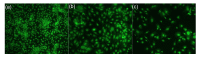
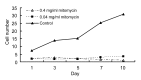
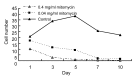
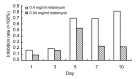
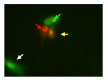
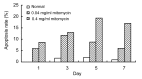
Results: The cultured normal human skin fibroblast and HaCat cell grew exponentially. A 5-min exposure to mitomycin at either 0.4 or 0.04 mg/ml caused marked dose-dependent cell proliferation inhibition on both fibroblasts and HaCat cells. Cell morphology changed, cell density decreased, and the growth curves were without an exponential phase. The fibroblast proliferated on the 5th day after the 5-min exposure of mitomycin at 0.04 mg/ml. Meanwhile, 5-min application of mitomycin at either 0.04 or 0.4 mg/ml induced fibroblast apoptosis but not necrosis. The apoptosis rate of the fibroblast increased with a higher concentration of mytomycin (p<0.05). A 5-min exposure to mitomycin at 0.4 mg/ml resulted in a marked decrease in the mRNA production of TGF-β1, procollagen I and III, and a marked increase in the mRNA production of bFGF.
Conclusions: Mitomycin can inhibit fibroblast proliferation, induce fibroblast apoptosis, and regulate intracellular protein expression on mRNA levels. In addition, mitomycin can inhibit HaCat cell proliferation, so epithelial cell needs more protecting to avoid mitomycin's side effect when it is applied clinically.
Figures


References
-
- Banthia V, Selesnick SH. Mitomycin-C in the Post Surgical Ear Canal. American Academy of Otolaryngology/Head and Neck Surgery Annual Meeting; Orlando, Florida, USA. 2003. pp. 882–886. - PubMed
-
- Battelino S, Hocevar-Boltezar I, Zargi M. Intraoperative use of mitomycin C in fibrous atresia of the external auditory canal. Ear Nose Throat J. 2005;84(12):776–779. - PubMed
-
- Crowston JG, Chang LH, Constable PH, Daniels JT, Akhar AN, Khaw PT. Apoptosis gene expression and death receptor signaling in mitomycin C-treated human Tenon capsule fibroblasts. Invest Ophthalmol Vis Sci. 2002;43(3):692–699. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

