Pharmacogenomics of drug metabolizing enzymes and transporters: implications for cancer therapy
- PMID: 23226051
- PMCID: PMC3513217
- DOI: 10.2147/PGPM.S18861
Pharmacogenomics of drug metabolizing enzymes and transporters: implications for cancer therapy
Abstract
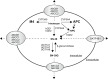
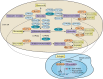
The new era of personalized medicine, which integrates the uniqueness of an individual with respect to the pharmacokinetics and pharmacodynamics of a drug, holds promise as a means to provide greater safety and efficacy in drug design and development. Personalized medicine is particularly important in oncology, whereby most clinically used anticancer drugs have a narrow therapeutic window and exhibit a large interindividual pharmacokinetic and pharmacodynamic variability. This variability can be explained, at least in part, by genetic variations in the genes encoding drug metabolizing enzymes, transporters, or drug targets. Understanding of how genetic variations influence drug disposition and action could help in tailoring cancer therapy based on individual's genetic makeup. This review focuses on the pharmacogenomics of drug metabolizing enzymes and drug transporters, with a particular highlight of examples whereby genetic variations in the metabolizing enzymes and transporters influence the pharmacokinetics and/or response of chemotherapeutic agents.
Keywords: drug; enzymes; oncology; personalized medicine; polymorphisms; transporters.
Figures

Similar articles
-
Pharmacogenomics of drug-metabolizing enzymes and drug transporters in chemotherapy.Methods Mol Biol. 2008;448:63-76. doi: 10.1007/978-1-59745-205-2_5. Methods Mol Biol. 2008. PMID: 18370231 Review.
-
Pharmacogenomics of Drug Metabolizing Enzymes and Transporters: Relevance to Precision Medicine.Genomics Proteomics Bioinformatics. 2016 Oct;14(5):298-313. doi: 10.1016/j.gpb.2016.03.008. Epub 2016 Oct 8. Genomics Proteomics Bioinformatics. 2016. PMID: 27729266 Free PMC article. Review.
-
Clinical pharmacogenetics and potential application in personalized medicine.Curr Drug Metab. 2008 Oct;9(8):738-84. doi: 10.2174/138920008786049302. Curr Drug Metab. 2008. PMID: 18855611 Review.
-
[Personalized dosing from perspective of pharmacogenomics of drug metabolizing enzymes and transporters].Yao Xue Xue Bao. 2017 Jan;52(1):1-7. Yao Xue Xue Bao. 2017. PMID: 29911367 Review. Chinese.
-
Pharmacogenomics: the inherited basis for interindividual differences in drug response.Annu Rev Genomics Hum Genet. 2001;2:9-39. doi: 10.1146/annurev.genom.2.1.9. Annu Rev Genomics Hum Genet. 2001. PMID: 11701642 Review.
Cited by
-
An Evolutionary Perspective on the Impact of Genomic Copy Number Variation on Human Health.J Mol Evol. 2020 Jan;88(1):104-119. doi: 10.1007/s00239-019-09911-6. Epub 2019 Sep 14. J Mol Evol. 2020. PMID: 31522275 Review.
-
Pharmacogenetics of Drugs Used in the Treatment of Cancers.Genes (Basel). 2022 Feb 7;13(2):311. doi: 10.3390/genes13020311. Genes (Basel). 2022. PMID: 35205356 Free PMC article. Review.
-
Effect of You-Gui Yin on the Activities of Seven Cytochrome P450 Isozymes in Rats.Evid Based Complement Alternat Med. 2020 May 13;2020:9784946. doi: 10.1155/2020/9784946. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32508959 Free PMC article.
-
Genetic Association of Olanzapine Treatment Response in Han Chinese Schizophrenia Patients.Front Pharmacol. 2019 Mar 4;10:177. doi: 10.3389/fphar.2019.00177. eCollection 2019. Front Pharmacol. 2019. PMID: 30886581 Free PMC article.
-
Correlations of CYP2C9*3/CYP2D6*10/CYP3A5*3 gene polymorphisms with efficacy of etanercept treatment for patients with ankylosing spondylitis: A case-control study.Medicine (Baltimore). 2017 Mar;96(9):e5993. doi: 10.1097/MD.0000000000005993. Medicine (Baltimore). 2017. PMID: 28248857 Free PMC article.
References
-
- Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. - PubMed
-
- Frueh FW, Amur S, Mummaneni P, et al. Pharmacogenomic biomarker information in drug labels approved by the United States food and drug administration: prevalence of related drug use. Pharmacotherapy. 2008;28:992–998. - PubMed
-
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229–243. - PubMed
-
- Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. - PubMed
-
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–1698. - PubMed
LinkOut - more resources
Full Text Sources

