Role of pharmacogenomics in the treatment of tuberculosis: a review
- PMID: 23226065
- PMCID: PMC3513231
- DOI: 10.2147/PGPM.S15454
Role of pharmacogenomics in the treatment of tuberculosis: a review
Abstract
Background: Tuberculosis is one of the major public health problems worldwide. Modern antituberculous treatment can cure most patients; cure rates > 95% are achieved with standard short-course chemotherapy regimens containing isoniazid, rifampicin, pyrazinamide, and ethambutol among patients with drug-susceptible strains of tuberculosis; however, a small proportion do not respond to treatment or develop serious adverse events. Pharmacogenomic studies of drugs used in the treatment of tuberculosis could help us understand intersubject variations in treatment response. In this review, we compiled pharmacogenomic data on antituberculous drugs that were available from different settings that would give a better insight into the role of pharmacogenomics in the treatment of tuberculosis, thereby enhancing the efficacy and limiting the toxicity of existing antituberculosis medications.
Methods: The PubMed database was searched from 1960 to the present using the keywords "tuberculosis", "antituberculosis treatment", "isoniazid", "rifampicin", "pyrazinamide", "ethambutol", "pharmacogenomics", and "polymorphism". Abstracts from meetings and review articles were included.
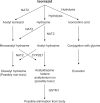
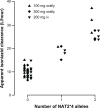
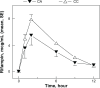
Conclusion: Studies conducted in different settings suggest that pharmacogenomics plays a significant role in isoniazid metabolism, and impacts both treatment efficacy and frequency of adverse reactions. Single nucleotide polymorphisms influencing plasma rifampicin concentrations are also reported. No data are available regarding other first-line drugs, ie, ethambutol and pyrazinamide. There is a need to incorporate pharmacogenomics into clinical trials of tuberculosis in order to understand the factors impacting therapeutic success and occurrence of adverse drug effects.
Keywords: antituberculous treatment; drug metabolism; pharmacogenomics; polymorphism; tuberculosis.
Figures

References
-
- World Health Organisation Global TB report. 2010.
-
- Crofton J. Global challenge of TB. Lancet. 1994;344(8922):609. - PubMed
-
- Vesell ES. Pharmacokinetic perspectives gained from twin and family studies. Pharmacol Ther. 1989;41(3):535–552. - PubMed
-
- Kalow W, Thang BK, Endreyi L. Hypothesis: comparisons of inter and intra-individual variations can substitute for twin studies in drug research. Pharmacokinetics. 1998;8(4):283–289. - PubMed
LinkOut - more resources
Full Text Sources

