Receptor-type Protein tyrosine phosphatase β regulates met phosphorylation and function in head and neck squamous cell carcinoma
- PMID: 23226095
- PMCID: PMC3514745
- DOI: 10.1593/neo.12870
Receptor-type Protein tyrosine phosphatase β regulates met phosphorylation and function in head and neck squamous cell carcinoma
Abstract
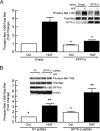
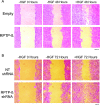
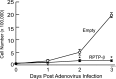
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer and has a high rate of mortality. Emerging evidence indicates that hepatocyte growth factor receptor (or Met) pathway plays a pivotal role in HNSCC metastasis and resistance to chemotherapy. Met function is dependent on tyrosine phosphorylation that is under direct control by receptor-type protein tyrosine phosphatase β (RPTP-β). We report here that RPTP-β expression is significantly downregulated in HNSCC cells derived from metastatic tumors compared to subject-matched cells from primary tumors. Knockdown of endogenous RPTP-β in HNSCC cells from primary tumor potentiated Met tyrosine phosphorylation, downstream mitogen-activated protein (MAP) kinase pathway activation, cell migration, and invasion. Conversely, restoration of RPTP-β expression in cells from matched metastatic tumor decreased Met tyrosine phosphorylation and downstream functions. Furthermore, we observed that six of eight HNSCC tumors had reduced levels of RPTP-β protein in comparison with normal oral tissues. Collectively, the results demonstrate the importance of RPTP-β in tumor biology of HNSCC through direct dephosphorylation of Met and regulation of downstream signal transduction pathways. Reduced RPTP-β levels, with or without Met overexpression, could promote Met activation in HNSCC tumors.
Figures




Similar articles
-
Receptor-type protein tyrosine phosphatase beta (RPTP-beta) directly dephosphorylates and regulates hepatocyte growth factor receptor (HGFR/Met) function.J Biol Chem. 2011 May 6;286(18):15980-8. doi: 10.1074/jbc.M110.212597. Epub 2011 Mar 15. J Biol Chem. 2011. PMID: 21454675 Free PMC article.
-
Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma.Cancer Res. 2001 Aug 1;61(15):5911-8. Cancer Res. 2001. PMID: 11479233
-
HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer.Clin Cancer Res. 2009 Jun 1;15(11):3740-50. doi: 10.1158/1078-0432.CCR-08-3252. Epub 2009 May 26. Clin Cancer Res. 2009. PMID: 19470725 Free PMC article.
-
C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell.Oral Oncol. 2014 Jul;50(7):633-9. doi: 10.1016/j.oraloncology.2014.04.004. Epub 2014 May 15. Oral Oncol. 2014. PMID: 24835851 Review.
-
Roles of the HGF/Met signaling in head and neck squamous cell carcinoma: Focus on tumor immunity (Review).Oncol Rep. 2020 Dec;44(6):2337-2344. doi: 10.3892/or.2020.7799. Epub 2020 Oct 9. Oncol Rep. 2020. PMID: 33125120 Review.
Cited by
-
Substrate specificity of R3 receptor-like protein-tyrosine phosphatase subfamily toward receptor protein-tyrosine kinases.J Biol Chem. 2013 Aug 9;288(32):23421-31. doi: 10.1074/jbc.M113.458489. Epub 2013 Jun 28. J Biol Chem. 2013. PMID: 23814054 Free PMC article.
-
Lipopolysaccharide-induced phosphorylation of c-Met tyrosine residue 1003 regulates c-Met intracellular trafficking and lung epithelial barrier function.Am J Physiol Lung Cell Mol Physiol. 2013 Jul 1;305(1):L56-63. doi: 10.1152/ajplung.00417.2012. Epub 2013 Apr 26. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23624790 Free PMC article.
-
Cancer subclonal genetic architecture as a key to personalized medicine.Neoplasia. 2013 Dec;15(12):1410-20. doi: 10.1593/neo.131972. Neoplasia. 2013. PMID: 24403863 Free PMC article.
-
Role of met axis in head and neck cancer.Cancers (Basel). 2013 Nov 26;5(4):1601-18. doi: 10.3390/cancers5041601. Cancers (Basel). 2013. PMID: 24287743 Free PMC article.
-
Physiological Signaling and Structure of the HGF Receptor MET.Biomedicines. 2014 Dec 31;3(1):1-31. doi: 10.3390/biomedicines3010001. Biomedicines. 2014. PMID: 28536396 Free PMC article. Review.
References
-
- De Herdt MJ, Baatenburg de Jong RJ. HGF and c-MET as potential orchestrators of invasive growth in head and neck squamous cell carcinoma. Front Biosci. 2008;13:2516–2526. - PubMed
-
- Hardisson D. Molecular pathogenesis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2003;260:502–508. - PubMed
-
- Rogers SJ, Harrington KJ, Rhys-Evans P, O-Charoenrat P, Eccles SA. Biological significance of c-erbB family oncogenes in head and neck cancer. Cancer Metastasis Rev. 2005;24:47–69. - PubMed
-
- Werner JA, Rathcke IO, Mandic R. The role of matrix metalloproteinases in squamous cell carcinomas of the head and neck. Clin Exp Metastasis. 2002;19:275–282. - PubMed
-
- Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
