Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell
- PMID: 23226119
- PMCID: PMC3508285
- DOI: 10.3389/fnbeh.2012.00082
Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell
Abstract
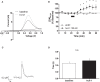
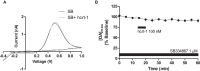
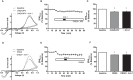
Hypocretins (hcrt), also known as orexins, play a critical role in reward-seeking behavior for natural rewards and drugs of abuse. The mesolimbic dopamine pathway that projects from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) is critically involved in the neural mechanisms underlying reward-seeking and motivation. Hcrt immunopositive fibers densely project to the shell of the nucleus accumbens (NAcSh), suggesting that the NAcSh might be a site for the interaction between hcrt and dopaminergic modulation of reward-seeking behavior. While it is known that hcrt action in the VTA can increase dopamine in the NAc, it has not been determined if hcrt released locally at dopaminergic terminals in the NAcSh can modulate dopamine concentration. Here, we use fast scan cyclic voltammetry (FSCV) in forebrain slices containing the NAcSh to determine whether hcrt can alter evoked dopamine concentration. We found bath application of hcrt-1 increases phasically evoked dopamine release, without altering reuptake at dopamine terminals in the NAcSh. Hcrt-1-induced potentiation of dopamine concentration was inhibited by SB334867, a hcrt receptor 1 antagonist, as well as ionotropic glutamate receptor antagonists, AP-5, CNQX and DNQX. Taken together, these results suggest that local hcrt-1 can modulate dopamine in the NAcSh and may play a role in reward-seeking and appetitive behaviors.
Keywords: dopamine; glutamate; hypocretin; nucleus accumbens shell; orexin; voltammetry.
Figures

References
LinkOut - more resources
Full Text Sources

