Transphosphorylation of E. coli Proteins during Production of Recombinant Protein Kinases Provides a Robust System to Characterize Kinase Specificity
- PMID: 23226150
- PMCID: PMC3510383
- DOI: 10.3389/fpls.2012.00262
Transphosphorylation of E. coli Proteins during Production of Recombinant Protein Kinases Provides a Robust System to Characterize Kinase Specificity
Abstract
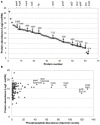
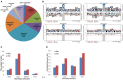
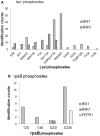
Protein kinase specificity is of fundamental importance to pathway regulation and signal transduction. Here, we report a convenient system to monitor the activity and specificity of recombinant protein kinases expressed in E. coli. We apply this to the study of the cytoplasmic domain of the plant receptor kinase BRASSINOSTEROID-INSENSITIVE 1 (BRI1), which functions in brassinosteroid (BR) signaling. Recombinant BRI1 is catalytically active and both autophosphorylates and transphosphorylates E. coli proteins in situ. Using enrichment approaches followed by LC-MS/MS, phosphosites were identified allowing motifs associated with auto- and transphosphorylation to be characterized. Four lines of evidence suggest that transphosphorylation of E. coli proteins by BRI1 is specific and therefore provides meaningful results: (1) phosphorylation is not correlated with bacterial protein abundance; (2) phosphosite stoichiometry, estimated by spectral counting, is also not related to protein abundance; (3) a transphosphorylation motif emerged with strong preference for basic residues both N- and C-terminal to the phosphosites; and (4) other protein kinases (BAK1, PEPR1, FLS2, and CDPKβ) phosphorylated a distinct set of E. coli proteins and phosphosites. The E. coli transphosphorylation assay can be applied broadly to protein kinases and provides a convenient and powerful system to elucidate kinase specificity.
Keywords: BAK1; BRI1; CDPK; FLS2; PEPR1; phosphorylation motif.
Figures





Similar articles
-
Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling.Dev Cell. 2008 Aug;15(2):220-35. doi: 10.1016/j.devcel.2008.06.011. Dev Cell. 2008. PMID: 18694562
-
Functional analysis of the BRI1 receptor kinase by Thr-for-Ser substitution in a regulatory autophosphorylation site.Front Plant Sci. 2015 Jul 30;6:562. doi: 10.3389/fpls.2015.00562. eCollection 2015. Front Plant Sci. 2015. PMID: 26284086 Free PMC article.
-
Functional importance of BAK1 tyrosine phosphorylation in vivo.Plant Signal Behav. 2011 Mar;6(3):400-5. doi: 10.4161/psb.6.3.14337. Epub 2011 Mar 1. Plant Signal Behav. 2011. Retraction in: Plant Signal Behav. 2016 Aug 2;11(8):e1211452. doi: 10.1080/15592324.2016.1211452. PMID: 21350342 Free PMC article. Retracted.
-
One for all: the receptor-associated kinase BAK1.Trends Plant Sci. 2009 Oct;14(10):535-41. doi: 10.1016/j.tplants.2009.08.002. Epub 2009 Sep 10. Trends Plant Sci. 2009. PMID: 19748302 Free PMC article. Review.
-
Novel Signaling Interface Constituted with Membrane Receptor-Like Kinases Emerged from the Study of Interaction and Transphosphorylation of BRI1 and BAK1.Curr Top Med Chem. 2017;17(21):2393-2400. doi: 10.2174/1568026617666170414144145. Curr Top Med Chem. 2017. PMID: 28413947 Review.
Cited by
-
The Carboxy-terminus of BAK1 regulates kinase activity and is required for normal growth of Arabidopsis.Front Plant Sci. 2014 Feb 4;5:16. doi: 10.3389/fpls.2014.00016. eCollection 2014. Front Plant Sci. 2014. Retraction in: Front Plant Sci. 2016 Jun 24;7:960. doi: 10.3389/fpls.2016.00960. PMID: 24550926 Free PMC article. Retracted.
-
Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation.Plant J. 2014 Apr;78(1):31-43. doi: 10.1111/tpj.12445. Epub 2014 Mar 12. Plant J. 2014. PMID: 24461462 Free PMC article.
-
VAMP726 and VAMP725 regulate vesicle secretion and pollen tube growth in Arabidopsis.Plant Cell Rep. 2023 Dec;42(12):1951-1965. doi: 10.1007/s00299-023-03075-w. Epub 2023 Oct 8. Plant Cell Rep. 2023. PMID: 37805949
-
Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks.Front Plant Sci. 2015 Nov 4;6:950. doi: 10.3389/fpls.2015.00950. eCollection 2015. Front Plant Sci. 2015. PMID: 26583025 Free PMC article. Review.
-
Regulation of Three Key Kinases of Brassinosteroid Signaling Pathway.Int J Mol Sci. 2020 Jun 18;21(12):4340. doi: 10.3390/ijms21124340. Int J Mol Sci. 2020. PMID: 32570783 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

