The impact of expanded testing for multidrug resistant tuberculosis using genotype [correction of geontype] MTBDRplus in South Africa: an observational cohort study
- PMID: 23226229
- PMCID: PMC3511489
- DOI: 10.1371/journal.pone.0049898
The impact of expanded testing for multidrug resistant tuberculosis using genotype [correction of geontype] MTBDRplus in South Africa: an observational cohort study
Erratum in
- PLoS One. 2013;8(6). doi: 10.1371/annotation/47420ec0-db00-4994-a0de-86b5e5d713f4 doi: 10.1371/annotation/47420ec0-db00-4994-a0de-86b5e5d713f4
Abstract
Introduction: Globally, multidrug resistant tuberculosis (MDR-TB) remains underdiagnosed. The Genotype MTBDRplus®, a rapid drug susceptibility testing (DST) assay used to detect resistance to isoniazid and rifampicin in the diagnosis of MDR-TB, has good diagnostic accuracy, but its impact on patient outcomes in routine practice is unproven. We assessed the clinical impact of routine DST using MTBDRplus in a single health district in South Africa.
Methods: Data were collected on all adult pulmonary TB patients registered at 25 public health clinics in the periods before and after introduction of an expanded DST algorithm using MTBDRplus version 1.0.
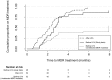
Results: We collected data on 1176 TB patients before implementation and 1177 patients afterwards. In the before period, measured MDR-TB prevalence among new cases was 0.7% (95% CI1.4-3.1%), and among retreatment cases 6.2% (95% CI:3.5-8.8%), versus 3.7% (95% CI:2.4-5.0, p<0.01) and 6.6% (95% CI:3.8-9.4%, p = 0.83) respectively after MTBDRplus introduction. The median times from sputum collection to MDR treatment in the before and after periods were 78 days (IQR:52-93) and 62 days (IQR:32-86, p = 0.05), respectively. Among MDR-TB cases, 27% (95%CI:10-44) in the before period converted sputum cultures to negative by 8 months following treatment initiation, while 52% (95%CI:38-66) converted in the intervention period (p = 0.04).
Conclusions: The expanded use of MTBDRplus DST resulted in a substantial increase in the proportion of new cases identified as MDR-TB; though time to MDR treatment was reduced, it was still over two months. Culture conversion for MDR-TB patients improved after introduction of MTBDRplus. This work illustrates the mixture of successes and challenges resulting from increased access to rapid DST in a setting with a high TB burden.
Conflict of interest statement
Figures




References
-
- WHO (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB), 2010 global report on surveillance and response. Geneva, Switzerland. - PubMed
-
- WHO (2011) Global Tuberculosis Control. Geneva.
-
- Ling DI, Zwerling AA, Pai M (2008) GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J 32: 1165–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

