Immunological and biochemical characterization of coxsackie virus A16 viral particles
- PMID: 23226233
- PMCID: PMC3511423
- DOI: 10.1371/journal.pone.0049973
Immunological and biochemical characterization of coxsackie virus A16 viral particles
Abstract
Background: Coxsackie virus A16 (CVA16) infections have become a serious public health problem in the Asia-Pacific region. It manifests most often in childhood exanthema, commonly known as hand-foot-and-mouth disease (HFMD). There are currently no vaccine or effective medical treatments available.
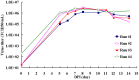
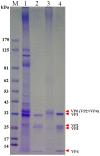
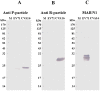
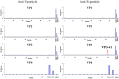
Principal finding: In this study, we describe the production, purification and characterization of CVA16 virus produced from Vero cells grown on 5 g/L Cytodex 1 microcarrier beads in a five-liter serum-free bioreactor system. The viral titer was found to be >10(6) the tissue culture's infectious dose (TCID(50)) per mL within 7 days post-infection when a multiplicity of infection (MOI) of 10(-5) was used for initial infection. Two CVA16 virus fractions were separated and detected when the harvested CVA16 viral concentrate was purified by a sucrose gradient zonal ultracentrifugation. The viral particles detected in the 24-28% sucrose fractions had low viral infectivity and RNA content. The viral particles obtained from 35-38% sucrose fractions were found to have high viral infectivity and RNA content, and composed of four viral proteins (VP1, VP2, VP3 and VP4), as shown by SDS-PAGE analyses. These two virus fractions were formalin-inactivated and only the infectious particle fraction was found to be capable of inducing CVA16-specific neutralizing antibody responses in both mouse and rabbit immunogenicity studies. But these antisera failed to neutralize enterovirus 71. In addition, rabbit antisera did not react with any peptides derived from CVA16 capsid proteins. Mouse antisera recognized a single linear immunodominant epitope of VP3 corresponding to residues 176-190.
Conclusion: These results provide important information for cell-based CVA16 vaccine development. To eliminate HFMD, a bivalent EV71/CVA16 vaccine formulation is necessary.
Conflict of interest statement
Figures

References
-
- McMinn PC (2002) An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 26: 91–107. - PubMed
-
- Schmidt NJ, Lennette EH, Ho HH (1974) An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 129: 304–9. - PubMed
-
- Ho M, Chen ER, Hsu KH, Wu SJT, Chen KT, et al. (1999) The Taiwan Enterovirus Epidemic Working Group. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med 341: 929–935. - PubMed
-
- Lin KH, Hwang KP, Ke GM, Wang CF, Ke LY, et al. (2006) Evolution of EV71 genogroup in Taiwan from 1998 to 2005: an emerging of subgenogroup C4 of EV71. J Med Virol 78: 254–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

