Identification of biomarkers of human skin ageing in both genders. Wnt signalling - a label of skin ageing?
- PMID: 23226273
- PMCID: PMC3511529
- DOI: 10.1371/journal.pone.0050393
Identification of biomarkers of human skin ageing in both genders. Wnt signalling - a label of skin ageing?
Abstract
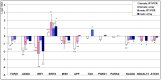
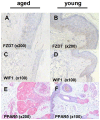
The goal of our work has been to investigate the mechanisms of gender-independent human skin ageing and examine the hypothesis of skin being an adequate model of global ageing. For this purpose, whole genome gene profiling was employed in sun-protected skin obtained from European Caucasian young and elderly females (mean age 26.7±4 years [n1 = 7] and 70.75±3.3 years [n2 = 4], respectively) and males (mean age 25.8±5.2 years [n3 = 6] and 76±3.8 years [n4 = 7], respectively) using the Illumina array platform. Confirmation of gene regulation was performed by real-time RT-PCR and immunohistochemistry. 523 genes were significantly regulated in female skin and 401 genes in male skin for the chosen criteria. Of these, 183 genes exhibited increased and 340 decreased expression in females whereas 210 genes showed increased and 191 decreased expression in males with age. In total, 39 genes were common in the target lists of significant regulated genes in males and females. 35 of these genes showed increased (16) or decreased (19) expression independent of gender. Only 4 overlapping genes (OR52N2, F6FR1OP2, TUBAL3 and STK40) showed differential regulation with age. Interestingly, Wnt signalling pathway showed to be significantly downregulated in aged skin with decreased gene and protein expression for males and females, accordingly. In addition, several genes involved in central nervous system (CNS) ageing (f.i. APP, TAU) showed to be expressed in human skin and were significanlty regulated with age. In conclusion, our study provides biomarkers of endogenous human skin ageing in both genders and highlight the role of Wnt signalling in this process. Furthermore, our data give evidence that skin could be used as a good alternative to understand ageing of different tissues such as CNS.
Conflict of interest statement
Figures


References
-
- Smith JR, Pereira-Smith OM (1996) Replicative senescence: implications for in vivo aging and tumor suppression. Science 273: 63–67. - PubMed
-
- Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G (1999) Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science 286: 774–779. - PubMed
-
- Miquel J (1998) An update on the oxygen stress-mitochondrial mutation theory of aging: genetic and evolutionary implications. Exp Gerontol 33: 113–126. - PubMed
-
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG (2000) Mitotic misregulation and human aging. Science 287: 2486–2492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

