Pre-treatment with allopurinol or uricase attenuates barrier dysfunction but not inflammation during murine ventilator-induced lung injury
- PMID: 23226314
- PMCID: PMC3511544
- DOI: 10.1371/journal.pone.0050559
Pre-treatment with allopurinol or uricase attenuates barrier dysfunction but not inflammation during murine ventilator-induced lung injury
Abstract
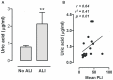
Introduction: Uric acid released from injured tissue is considered a major endogenous danger signal and local instillation of uric acid crystals induces acute lung inflammation via activation of the NLRP3 inflammasome. Ventilator-induced lung injury (VILI) is mediated by the NLRP3 inflammasome and increased uric acid levels in lung lavage fluid are reported. We studied levels in human lung injury and the contribution of uric acid in experimental VILI.
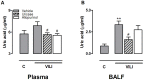
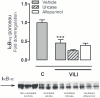
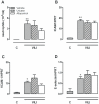
Methods: Uric acid levels in lung lavage fluid of patients with acute lung injury (ALI) were determined. In a different cohort of cardiac surgery patients, uric acid levels were correlated with pulmonary leakage index. In a mouse model of VILI the effect of allopurinol (inhibits uric acid synthesis) and uricase (degrades uric acid) pre-treatment on neutrophil influx, up-regulation of adhesion molecules, pulmonary and systemic cytokine levels, lung pathology, and regulation of receptors involved in the recognition of uric acid was studied. In addition, total protein and immunoglobulin M in lung lavage fluid and pulmonary wet/dry ratios were measured as markers of alveolar barrier dysfunction.
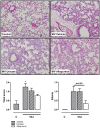
Results: Uric acid levels increased in ALI patients. In cardiac surgery patients, elevated levels correlated significantly with the pulmonary leakage index. Allopurinol or uricase treatment did not reduce ventilator-induced inflammation, IκB-α degradation, or up-regulation of NLRP3, Toll-like receptor 2, and Toll-like receptor 4 gene expression in mice. Alveolar barrier dysfunction was attenuated which was most pronounced in mice pre-treated with allopurinol: both treatment strategies reduced wet/dry ratio, allopurinol also lowered total protein and immunoglobulin M levels.
Conclusions: Local uric acid levels increase in patients with ALI. In mice, allopurinol and uricase attenuate ventilator-induced alveolar barrier dysfunction.
Conflict of interest statement
Figures



Similar articles
-
Ventilator-induced lung injury is mediated by the NLRP3 inflammasome.Anesthesiology. 2012 May;116(5):1104-15. doi: 10.1097/ALN.0b013e3182518bc0. Anesthesiology. 2012. PMID: 22531249
-
Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis.Am J Respir Crit Care Med. 2009 May 15;179(10):903-13. doi: 10.1164/rccm.200808-1274OC. Epub 2009 Feb 12. Am J Respir Crit Care Med. 2009. PMID: 19218193
-
Lipoxin A4 Reduces Ventilator-Induced Lung Injury in Rats with Large-Volume Mechanical Ventilation.Mediators Inflamm. 2020 Nov 22;2020:6705985. doi: 10.1155/2020/6705985. eCollection 2020. Mediators Inflamm. 2020. PMID: 33299377 Free PMC article.
-
Regulation of cell proliferation and transdifferentiation compensates for ventilator-induced lung injury mediated by NLRP3 inflammasome activation.Immun Inflamm Dis. 2023 Oct;11(10):e1062. doi: 10.1002/iid3.1062. Immun Inflamm Dis. 2023. PMID: 37904713 Free PMC article. Review.
-
Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury.Crit Care. 2011;15(6):235. doi: 10.1186/cc10437. Epub 2011 Nov 30. Crit Care. 2011. PMID: 22216838 Free PMC article. Review.
Cited by
-
Uricase Inhibits Nitrogen Dioxide-Promoted Allergic Sensitization to Inhaled Ovalbumin Independent of Uric Acid Catabolism.J Immunol. 2016 Sep 1;197(5):1720-32. doi: 10.4049/jimmunol.1600336. Epub 2016 Jul 27. J Immunol. 2016. PMID: 27465529 Free PMC article.
-
Dexamethasone attenuates VEGF expression and inflammation but not barrier dysfunction in a murine model of ventilator-induced lung injury.PLoS One. 2013;8(2):e57374. doi: 10.1371/journal.pone.0057374. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451215 Free PMC article.
-
Circadian rhythm reprogramming during lung inflammation.Nat Commun. 2014 Sep 11;5:4753. doi: 10.1038/ncomms5753. Nat Commun. 2014. PMID: 25208554 Free PMC article.
-
Neutrophil Extracellular Traps Stimulate Proinflammatory Responses in Human Airway Epithelial Cells.J Innate Immun. 2017;9(4):387-402. doi: 10.1159/000460293. Epub 2017 May 4. J Innate Immun. 2017. PMID: 28467984 Free PMC article.
-
The Role of Epithelial Damage in the Pulmonary Immune Response.Cells. 2021 Oct 15;10(10):2763. doi: 10.3390/cells10102763. Cells. 2021. PMID: 34685744 Free PMC article. Review.
References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA. 20 307: 2526–33. - PubMed
-
- dos Santos CC, Slutsky AS (2006) The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 68: 585–618. - PubMed
-
- The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308. - PubMed
-
- Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, et al. (2011) Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM: The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 37: 1932–1941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

