Non-invasive imaging of endothelial progenitor cells in tumor neovascularization using a novel dual-modality paramagnetic/near-infrared fluorescence probe
- PMID: 23226317
- PMCID: PMC3511537
- DOI: 10.1371/journal.pone.0050575
Non-invasive imaging of endothelial progenitor cells in tumor neovascularization using a novel dual-modality paramagnetic/near-infrared fluorescence probe
Abstract
Objective: Bone-marrow derived endothelial progenitor cells (EPCs) play an important role in tumor neovasculature. Due to their tumor homing property, EPCs are regarded as promising targeted vectors for delivering therapeutic agents in cancer treatment. Consequently, non-invasive confirmation of targeted delivery via imaging is urgently needed. This study shows the development and application of a novel dual-modality probe for in vivo non-invasively tracking of the migration, homing and differentiation of EPCs.
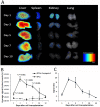
Methods: The paramagnetic/near-infrared fluorescence probe Conjugate 1 labeled EPCs were systemically transplanted into mice bearing human breast MDA-MB-231 tumor xenografts. Magnetic resonance imaging (MRI) and near-infrared (NIR) fluorescence optical imaging were performed at different stages of tumor development. The homing of EPCs and the tumor neovascularization were further evaluated by immunofluorescence.
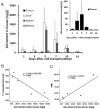
Results: Conjugate 1 labeled EPCs can be monitored in vivo by MRI and NIR fluorescence optical imaging without altering tumor growth for up to three weeks after the systemic transplantation. Histopathological examination confirmed that EPCs were recruited into the tumor bed and then incorporated into new vessels two weeks after the transplantation. Tumor size and microvessel density was not influenced by EPCs transplantation in the first three weeks.
Conclusions: This preclinical study shows the feasibility of using a MRI and NIR fluorescence optical imaging detectable probe to non-invasively monitor transplanted EPCs and also provides strong evidence that EPCs are involved in the development of endothelial cells during the tumor neovascularization.
Conflict of interest statement
Figures




References
-
- Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228. - PubMed
-
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, et al. (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7: 1194–1201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

