Genotoxic agents promote the nuclear accumulation of annexin A2: role of annexin A2 in mitigating DNA damage
- PMID: 23226323
- PMCID: PMC3511559
- DOI: 10.1371/journal.pone.0050591
Genotoxic agents promote the nuclear accumulation of annexin A2: role of annexin A2 in mitigating DNA damage
Abstract
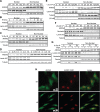
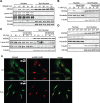
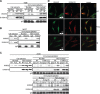
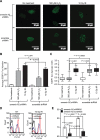
Annexin A2 is an abundant cellular protein that is mainly localized in the cytoplasm and plasma membrane, however a small population has been found in the nucleus, suggesting a nuclear function for the protein. Annexin A2 possesses a nuclear export sequence (NES) and inhibition of the NES is sufficient to cause nuclear accumulation. Here we show that annexin A2 accumulates in the nucleus in response to genotoxic agents including gamma-radiation, UV radiation, etoposide and chromium VI and that this event is mediated by the nuclear export sequence of annexin A2. Nuclear accumulation of annexin A2 is blocked by the antioxidant agent N-acetyl cysteine (NAC) and stimulated by hydrogen peroxide (H₂O₂), suggesting that this is a reactive oxygen species dependent event. In response to genotoxic agents, cells depleted of annexin A2 show enhanced phospho-histone H2AX and p53 levels, increased numbers of p53-binding protein 1 nuclear foci and increased levels of nuclear 8-oxo-2'-deoxyguanine, suggesting that annexin A2 plays a role in protecting DNA from damage. This is the first report showing the nuclear translocation of annexin A2 in response to genotoxic agents and its role in mitigating DNA damage.
Conflict of interest statement
Figures



References
-
- Gerke V, Creutz CE, Moss SE (2005) Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6: 449–461. - PubMed
-
- Waisman DM (1995) Annexin II tetramer: structure and function. Mol Cell Biochem 149–150: 301–322. - PubMed
-
- Jost M, Thiel C, Weber K, Gerke V (1992) Mapping of three unique Ca(2+)-binding sites in human annexin II. Eur J Biochem 207: 923–930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

