Cell therapy: a safe and efficacious therapeutic treatment for Alzheimer's disease in APP+PS1 mice
- PMID: 23226497
- PMCID: PMC3513317
- DOI: 10.1371/journal.pone.0049468
Cell therapy: a safe and efficacious therapeutic treatment for Alzheimer's disease in APP+PS1 mice
Erratum in
-
Correction: Cell Therapy: A Safe and Efficacious Therapeutic Treatment for Alzheimer's Disease in APP+PS1 Mice.PLoS One. 2024 May 9;19(5):e0303619. doi: 10.1371/journal.pone.0303619. eCollection 2024. PLoS One. 2024. PMID: 38722875 Free PMC article.
Abstract
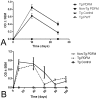
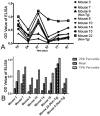
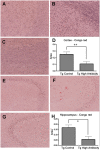
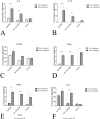
Previously, our lab was the first to report the use of antigen-sensitized dendritic cells as a vaccine against Alzheimer's disease (AD). In preparation of this vaccine, we sensitized the isolated dendritic cells ex vivo with Aβ peptide, and administered these sensitized dendritic cells as a therapeutic agent. This form of cell therapy has had success in preventing and/or slowing the rate of cognitive decline when administered prior to the appearance of Aβ plaques in PDAPP mice, but has not been tested in 2 × Tg models. Herein, we test the efficacy and safety of this vaccine in halting and reversing Alzheimer's pathology in 9-month-old APP + PS1 mice. The results showed that administration of this vaccine elicits a long-lasting antibody titer, which correlated well with a reduction of Aβ burden upon histological analysis. Cognitive function in transgenic responders to the vaccine was rescued to levels similar to those found in non-transgenic mice, indicating that the vaccine is capable of providing therapeutic benefit in APP+PS1 mice when administered after the onset of AD pathology. The vaccine also shows indications of circumventing past safety problems observed in AD immunotherapy, as Th1 pro-inflammatory cytokines were not elevated after long-term vaccine administration. Moreover, microhemorrhaging and T-cell infiltration into the brain are not observed in any of the treated subjects. All in all, this vaccine has many advantages over contemporary vaccines against Alzheimer's disease, and may lead to a viable treatment for the disease in the future.
Conflict of interest statement
Figures






References
-
- Association As (2012) Alzheimer’s disease facts and figures. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. 131–168. - PubMed
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. United States. 186–191. - PubMed
-
- Ja H, Ga H (1992) - Alzheimer’s disease: the amyloid cascade hypothesis. Science 256: 184–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

