E4 antibodies facilitate detection and type-assignment of active HPV infection in cervical disease
- PMID: 23226504
- PMCID: PMC3513315
- DOI: 10.1371/journal.pone.0049974
E4 antibodies facilitate detection and type-assignment of active HPV infection in cervical disease
Abstract
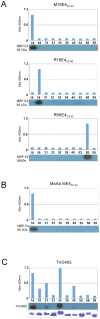
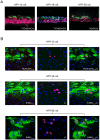
High-risk human papillomavirus (HPV) infections are the cause of nearly all cases of cervical cancer. Although the detection of HPV DNA has proved useful in cervical diagnosis, it does not necessarily predict disease presence or severity, and cannot conclusively identify the causative type when multiple HPVs are present. Such limitations may be addressed using complementary approaches such as cytology, laser capture microscopy, and/or the use of infection biomarkers. One such infection biomarker is the HPV E4 protein, which is expressed at high level in cells that are supporting (or have supported) viral genome amplification. Its distribution in lesions has suggested a role in disease staging. Here we have examined whether type-specific E4 antibodies may also allow the identification and/or confirmation of causal HPV-type. To do this, type-specific polyclonal and monoclonal antibodies against three E4 proteins (HPV-16, -18, and -58) were generated and validated by ELISA and western blotting, and by immunohistochemistry (IHC) staining of epithelial rafts containing these individual HPV types. Type-specific detection of HPV and its associated disease was subsequently examined using formalin-fixed paraffin-embedded cervical intra-epithelial neoplasias (CIN, (n = 247)) and normal controls (n = 28). All koilocytotic CIN1 lesions showed type-specific E4 expression of their respective HPV types. Differences were noted amongst E4 expression patterns in CIN3. HPV-18 E4 was not detected in any of the 6 HPV-18 DNA-positive CIN3 lesions examined, whereas in HPV-16 and -58 CIN3, 28/37 (76%) and 5/9 (55.6%) expressed E4 respectively, usually in regions of epithelial differentiation. Our results demonstrate that type-specific E4 antibodies can be used to help establish causality, as may be required when multiple HPV types are detected. The unique characteristics of the E4 biomarker suggest a role in diagnosis and patient management particularly when used in combination.
Conflict of interest statement
Figures





References
-
- Walboomers J, Jacobs M, Manos MM, Bosch F, Kummer J, et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19. - PubMed
-
- Wentzensen N, Hampl M, Herkert M, Reichert A, Trunk MJ, et al. (2006) Identification of high-grade cervical dysplasia by the detection of p16INK4a in cell lysates obtained from cervical samples. Cancer 107: 2307–2313. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

