Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques
- PMID: 23226776
- PMCID: PMC3494114
- DOI: 10.3892/etm.2012.722
Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques
Abstract
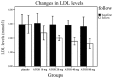
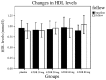
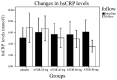
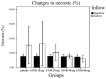
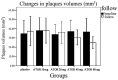
Statin treatment in patients with coronary heart disease is associated with a reduced incidence of short-term adverse events and endpoint cardiac events. However, the effects of statin treatment on atherosclerotic plaques, particularly stable plaques, remain poorly defined. In total, 228 consecutive patients with stable atherosclerotic plaques who had undergone coronary arteriography (CAG) and intravascular ultrasound (IVUS) were randomly assigned to receive placebo (placebo group, n=54) or atorvastatin (ATOR) at a single daily dose of 10 mg (ATOR 10 mg group, n=47), 20 mg (ATOR 20 mg group, n=45), 40 mg (ATOR 40 mg group, n=43) or 80 mg (ATOR 80 mg group, n=39). Endpoints, including serum lipids, serum inflammation, plaque volume and percentage of plaque necrosis were assessed after 3-6 months. At baseline, mean low-density lipoprotein (LDL), high-density lipoprotein (HDL) and high-sensitivity C-reactive protein (hs-CRP) levels, as well as plaque volumes and percentages of plaque necrosis, were similar between all groups. At 6 months of follow-up, the LDL levels in the ATOR groups were below those at their respective baselines (P<0.01). HDL levels in the ATOR 80 mg group following treatment were significantly higher compared with baseline (P=0.001). Additionally, they were significantly higher compared with those in the placebo, ATOR 10, 20 and 40 mg groups (P<0.01, P=0.001, P=0.048, P=0.047, respectively). Hs-CRP levels in the placebo group following treatment were higher compared with baseline levels (6.87±2.62 vs. 5.07±1.80, P<0.01), but hs-CRP levels in the ATOR 80 mg group following treatment were lower compared with baseline (3.59±1.07 vs. 6.10±2.12, P<0.01). According to the virtual histology (VH) of IVUS, the percentages of plaque necrosis following treatment in the placebo and ATOR 10 mg groups rose above baseline levels (15.51±12.56 vs. 7.69±1.31%, 13.54±11.76 vs. 7.83±1.43%, P<0.01) and conformed to the diagnostic criteria for unstable plaques (15.51±12.56, 13.54±11.76%). By contrast, in the ATOR 20, 40 and 80 mg groups, percentages of plaque necrosis remained stable following treatment compared with baseline (P=0.069, 0.846 and 0.643, respectively). Plaque volumes following treatment in the placebo, ATOR 10 and 20 mg groups were similar to baseline levels. However, in the ATOR 40 and 80 mg groups, plaque volumes decreased following treatment compared with baseline plaque volumes (30.69±8.12 vs. 37.09±12.01 mm(3), 24.99±1.01 vs. 36.47±14.68 mm(3), P=0.019, P<0.01, respectively). ATOR (20 mg/day) is able to lower LDL to standard levels while ATOR 40 mg/day was superior to 20 mg/day and had similar effects to 80 mg/day. Only ATOR 80 mg/day was able to increase HDL levels. Hs-CRP in patients without ATOR was higher and ATOR 80 mg/day decreased levels. ATOR ≥20 mg/day is able to stabilize plaques and ATOR 80 mg/day was superior to 20 and 40 mg/day. Thus, ATOR 40-80 mg/day reduces the volume of plaques.
Figures
References
-
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. - PubMed
-
- Ray KK, Cannon CP, McCabe CH, et al. PROVE IT-TIMI 22 Investigators Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2005;46:1405–1410. - PubMed
-
- Ishizu T, Seo Y, Machino T, et al. Prognostic impact of plaque echolucency in combination with inflammatory biomarkers on cardiovascular outcomes of coronary artery disease patients receiving optimal medical therapy. Atherosclerosis. 2011;216:120–124. - PubMed
-
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. - PubMed
-
- Yokoi H, Nobuyoshi M, Mitsudo K, Kawaguchi A, Yamamoto A, ATHEROMA Study Investigators Three-year follow-up results of angiographic intervention trial using an HMG-CoA reductase inhibitor to evaluate retardation of obstructive multiple atheroma (ATHEROMA) study. Circ J. 2005;69:875–883. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
