Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review)
- PMID: 23226794
- PMCID: PMC3506713
- DOI: 10.3892/ol.2012.928
Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review)
Abstract
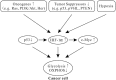
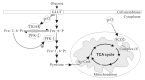
Metabolic activities in normal cells rely primarily on mitochondrial oxidative phosphorylation (OXPHOS) to generate ATP for energy. Unlike in normal cells, glycolysis is enhanced and OXPHOS capacity is reduced in various cancer cells. It has long been believed that the glycolytic phenotype in cancer is due to a permanent impairment of mitochondrial OXPHOS, as proposed by Otto Warburg. This view is challenged by recent investigations which find that the function of mitochondrial OXPHOS in most cancers is intact. Aerobic glycolysis in many cancers is the combined result of various factors such as oncogenes, tumor suppressors, a hypoxic microenvironment, mtDNA mutations, genetic background and others. Understanding the features and complexity of the cancer energy metabolism will help to develop new approaches in early diagnosis and effectively target therapy of cancer.
Figures
References
-
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. - PubMed
-
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. - PubMed
-
- Griguer CE, Oliva CR, Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol. 2005;74:123–133. - PubMed
-
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. - PubMed
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
