Energy metabolism of the visual system
- PMID: 23226947
- PMCID: PMC3515641
- DOI: 10.2147/EB.S9078
Energy metabolism of the visual system
Abstract
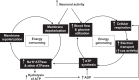
The visual system is one of the most energetically demanding systems in the brain. The currency of energy is ATP, which is generated most efficiently from oxidative metabolism in the mitochondria. ATP supports multiple neuronal functions. Foremost is repolarization of the membrane potential after depolarization. Neuronal activity, ATP generation, blood flow, oxygen consumption, glucose utilization, and mitochondrial oxidative metabolism are all interrelated. In the retina, phototransduction, neurotransmitter utilization, and protein/organelle transport are energy-dependent, yet repolarization-after-depolarization consumes the bulk of the energy. Repolarization in photoreceptor inner segments maintains the dark current. Repolarization by all neurons along the visual pathway following depolarizing excitatory glutamatergic neurotransmission preserves cellular integrity and permits reactivation. The higher metabolic activity in the magno- versus the parvo-cellular pathway, the ON- versus the OFF-pathway in some (and the reverse in other) species, and in specialized functional representations in the visual cortex all reflect a greater emphasis on the processing of specific visual attributes. Neuronal activity and energy metabolism are tightly coupled processes at the cellular and even at the molecular levels. Deficiencies in energy metabolism, such as in diabetes, mitochondrial DNA mutation, mitochondrial protein malfunction, and oxidative stress can lead to retinopathy, visual deficits, neuronal degeneration, and eventual blindness.
Conflict of interest statement
The author reports no conflicts of interest in this work.
Figures







References
-
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. - PubMed
-
- Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott-Raven; 1999. pp. 637–669.
-
- Weber B, Keller AL, Reichold J, Logothetis NK. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb Cortex. 2008;18:2318–2330. - PubMed
-
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. - PubMed
-
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Reviews. 2000;34:42–68. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

