Presynaptic GABA(B) Receptor Regulates Activity-Dependent Maturation and Patterning of Inhibitory Synapses through Dynamic Allocation of Synaptic Vesicles
- PMID: 23227002
- PMCID: PMC3512030
- DOI: 10.3389/fncel.2012.00057
Presynaptic GABA(B) Receptor Regulates Activity-Dependent Maturation and Patterning of Inhibitory Synapses through Dynamic Allocation of Synaptic Vesicles
Abstract
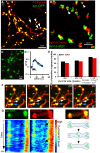
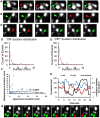
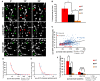
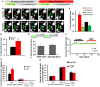
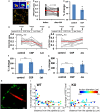
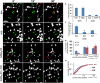
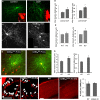
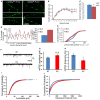
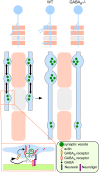
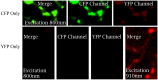
Accumulating evidence indicate that GABA regulates activity-dependent development of inhibitory synapses in the vertebrate brain, but the underlying mechanisms remain unclear. Here we combined live imaging of cortical GABAergic axons with single cell genetic manipulation to dissect the role of presynaptic GABA(B) receptors (GABA(B)Rs) in inhibitory synapse formation in mouse. Developing GABAergic axons form a significant number of transient boutons but only a subset was stabilized. Synaptic vesicles in these nascent boutons are often highly mobile in the course of tens of minutes. Activation of presynaptic GABA(B)Rs stabilized mobile vesicles in nascent boutons through the local enhancement of actin polymerization. Inactivation of GABA(B)Rs in developing basket interneurons resulted in aberrant pattern of bouton size distribution, reduced bouton density and reduced axon branching, as well as reduced frequency of miniature inhibitory currents in postsynaptic pyramidal neurons. These results suggest that GABA(B)Rs along developing inhibitory axons act as a local sensor of GABA release and promote presynaptic maturation through increased recruitment of mobile vesicle pools. Such release-dependent validation and maturation of nascent terminals is well suited to sculpt the pattern of synapse formation and distribution along axon branches.
Keywords: FRET; GABAB receptor; actin polymerization; activity-dependent development; inhibitory synapses; live cell imaging; synaptic vesicle dynamics.
Figures
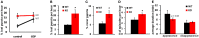
Similar articles
-
GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons.J Neurosci. 2012 Jan 4;32(1):331-43. doi: 10.1523/JNEUROSCI.3189-11.2012. J Neurosci. 2012. PMID: 22219294 Free PMC article.
-
Inhibitory synapse dynamics: coordinated presynaptic and postsynaptic mobility and the major contribution of recycled vesicles to new synapse formation.J Neurosci. 2011 Jul 20;31(29):10481-93. doi: 10.1523/JNEUROSCI.6023-10.2011. J Neurosci. 2011. PMID: 21775594 Free PMC article.
-
Semaphorin4D Induces Inhibitory Synapse Formation by Rapid Stabilization of Presynaptic Boutons via MET Coactivation.J Neurosci. 2019 May 29;39(22):4221-4237. doi: 10.1523/JNEUROSCI.0215-19.2019. Epub 2019 Mar 26. J Neurosci. 2019. PMID: 30914448 Free PMC article.
-
GABAB receptors: modulation of thalamocortical dynamics and synaptic plasticity.Neuroscience. 2021 Feb 21;456:131-142. doi: 10.1016/j.neuroscience.2020.03.011. Epub 2020 Mar 17. Neuroscience. 2021. PMID: 32194227 Review.
-
Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond.J Physiol. 2009 May 1;587(Pt 9):1881-8. doi: 10.1113/jphysiol.2008.168211. Epub 2009 Feb 2. J Physiol. 2009. PMID: 19188247 Free PMC article. Review.
Cited by
-
Mature parvalbumin interneuron function in prefrontal cortex requires activity during a postnatal sensitive period.Elife. 2022 Dec 28;11:e80324. doi: 10.7554/eLife.80324. Elife. 2022. PMID: 36576777 Free PMC article.
-
Molecular Mechanisms of L1 and NCAM Adhesion Molecules in Synaptic Pruning, Plasticity, and Stabilization.Front Cell Dev Biol. 2021 Jan 28;9:625340. doi: 10.3389/fcell.2021.625340. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585481 Free PMC article. Review.
-
Modulation of synaptic function through the α-neurexin-specific ligand neurexophilin-1.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):E1274-83. doi: 10.1073/pnas.1312112111. Epub 2014 Mar 17. Proc Natl Acad Sci U S A. 2014. PMID: 24639499 Free PMC article.
-
Structural plasticity of GABAergic axons is regulated by network activity and GABAA receptor activation.Front Neural Circuits. 2013 Jun 26;7:113. doi: 10.3389/fncir.2013.00113. eCollection 2013. Front Neural Circuits. 2013. PMID: 23805077 Free PMC article.
-
Loss-of-Huntingtin in Medial and Lateral Ganglionic Lineages Differentially Disrupts Regional Interneuron and Projection Neuron Subtypes and Promotes Huntington's Disease-Associated Behavioral, Cellular, and Pathological Hallmarks.J Neurosci. 2019 Mar 6;39(10):1892-1909. doi: 10.1523/JNEUROSCI.2443-18.2018. Epub 2019 Jan 9. J Neurosci. 2019. PMID: 30626701 Free PMC article.
References
LinkOut - more resources
Full Text Sources

