Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits
- PMID: 23227003
- PMCID: PMC3514612
- DOI: 10.3389/fncir.2012.00082
Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits
Abstract
Nitric oxide (NO) is an important signaling molecule crucial for many physiological processes such as synaptic plasticity, vasomotricity, and inflammation. Neuronal nitric oxide synthase (nNOS) is the enzyme responsible for the synthesis of NO by neurons. In the juvenile and mature hippocampus and neocortex nNOS is primarily expressed by subpopulations of GABAergic interneurons. Over the past two decades, many advances have been achieved in the characterization of neocortical and hippocampal nNOS expressing neurons. In this review, we summarize past and present studies that have characterized the electrophysiological, morphological, molecular, and synaptic properties of these neurons. We also discuss recent studies that have shed light on the developmental origins and specification of GABAergic neurons with specific attention to neocortical and hippocampal nNOS expressing GABAergic neurons. Finally, we summarize the roles of NO and nNOS-expressing inhibitory neurons.
Keywords: GABA; classification; development; interneurons; nNOS; specification.
Figures

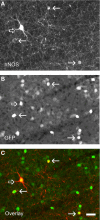
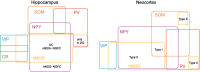
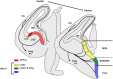
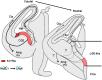
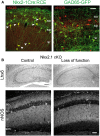
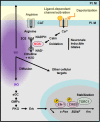
References
LinkOut - more resources
Full Text Sources
Miscellaneous

