Regulation of transport in the connecting tubule and cortical collecting duct
- PMID: 23227301
- PMCID: PMC3516049
- DOI: 10.1002/cphy.c110052
Regulation of transport in the connecting tubule and cortical collecting duct
Abstract
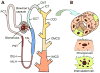
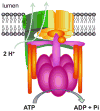
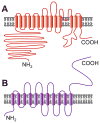
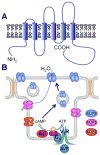
The central goal of this overview article is to summarize recent findings in renal epithelial transport,focusing chiefly on the connecting tubule (CNT) and the cortical collecting duct (CCD).Mammalian CCD and CNT are involved in fine-tuning of electrolyte and fluid balance through reabsorption and secretion. Specific transporters and channels mediate vectorial movements of water and solutes in these segments. Although only a small percent of the glomerular filtrate reaches the CNT and CCD, these segments are critical for water and electrolyte homeostasis since several hormones, for example, aldosterone and arginine vasopressin, exert their main effects in these nephron sites. Importantly, hormones regulate the function of the entire nephron and kidney by affecting channels and transporters in the CNT and CCD. Knowledge about the physiological and pathophysiological regulation of transport in the CNT and CCD and particular roles of specific channels/transporters has increased tremendously over the last two decades.Recent studies shed new light on several key questions concerning the regulation of renal transport.Precise distribution patterns of transport proteins in the CCD and CNT will be reviewed, and their physiological roles and mechanisms mediating ion transport in these segments will also be covered. Special emphasis will be given to pathophysiological conditions appearing as a result of abnormalities in renal transport in the CNT and CCD.
© 2012 American Physiological Society. Compr Physiol 2:1491-1539, 2012.
Figures












References
-
- Adachi S, Uchida S, Ito H, Hata M, Hiroe M, Marumo F, Sasaki S. Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle’s loop and collecting ducts of rat kidney. J Biol Chem. 1994;269:17677–17683. - PubMed
-
- Ahn KY, Turner PB, Madsen KM, Kone BC. Effects of chronic hypokalemia on renal expression of the “gastric” H+,K+-ATPase alpha-subunit gene. Am J Physiol Renal Physiol. 1996;270:F557–F566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

