Antigenicity and Immunogenicity in HIV-1 Antibody-Based Vaccine Design
- PMID: 23227445
- PMCID: PMC3515071
- DOI: 10.4172/2155-6113
Antigenicity and Immunogenicity in HIV-1 Antibody-Based Vaccine Design
Abstract
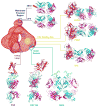
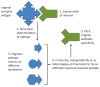
Neutralizing antibodies can protect from infection by immunodeficiency viruses. However, the induction by active vaccination of antibodies that can potently neutralize a broad range of circulating virus strains is a goal not yet achieved, despite more than 2 decades of research. Here we review progress made in the field, from early empirical studies to today's rational structure-based vaccine antigen design. We discuss the existence of broadly neutralizing antibodies, their implications for epitope discovery and recent progress made in antigen design. Finally, we consider the relationship between antigenicity and immunogenicity for B cell recognition and antibody production, a major hurdle for rational vaccine design to overcome.
Figures
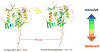
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
