Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats
- PMID: 23227953
- PMCID: PMC3636588
- DOI: 10.1089/neu.2012.2782
Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats
Abstract
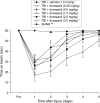
The acetylcholinesterase (AChE) inhibitor donepezil is used as a therapy for Alzheimer's disease and has been recommended as a treatment for enhancing attention and memory after traumatic brain injury (TBI). Although select clinical case studies support the use of donepezil for enhancing cognition, there is a paucity of experimental TBI studies assessing the potential efficacy of this pharmacotherapy. Hence, the aim of this pre-clinical study was to evaluate several doses of donepezil to determine its effect on functional outcome after TBI. Ninety anesthetized adult male rats received a controlled cortical impact (CCI; 2.8 mm cortical depth at 4 m/sec) or sham injury, and then were randomly assigned to six TBI and six sham groups (donepezil 0.25, 0.5, 1.0, 2.0, or 3.0 mg/kg, and saline vehicle 1.0 mL/kg). Treatments began 24 h after surgery and were administered i.p. once daily for 19 days. Function was assessed by motor (beam balance/walk) and cognitive (Morris water maze) tests on days 1-5 and 14-19, respectively. No significant differences were observed among the sham control groups in any evaluation, regardless of dose, and therefore the data were pooled. Furthermore, no significant differences were revealed among the TBI groups in acute neurological assessments (e.g., righting reflex), suggesting that all groups received the same level of injury severity. None of the five doses of donepezil improved motor or cognitive function relative to vehicle-treated controls. Moreover, the two highest doses significantly impaired beam-balance (3.0 mg/kg), beam-walk (2.0 mg/kg and 3.0 mg/kg), and cognitive performance (3.0 mg/kg) versus vehicle. These data indicate that chronic administration of donepezil is not only ineffective in promoting functional improvement after moderate CCI injury, but depending on the dose is actually detrimental to the recovery process. Further work is necessary to determine if other AChE inhibitors exert similar effects after TBI.
Figures


References
-
- Centers for Disease Control and Prevention (CDC) National Center for Injury Prevention and Control (2003) Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention; Atlanta:
-
- Moore E.L. Terryberry-Spohr L. Hope D.A. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 2006;20:117–132. - PubMed
-
- Selassie A.W. Zaloshnja E. Langlois J.A. Miller T. Jones P. Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. - PubMed
-
- Summers C.R. Ivins B. Schwab K.A. Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 2009;76:105–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

