HIV-1 Nef: a multifaceted modulator of T cell receptor signaling
- PMID: 23227982
- PMCID: PMC3534016
- DOI: 10.1186/1478-811X-10-39
HIV-1 Nef: a multifaceted modulator of T cell receptor signaling
Abstract
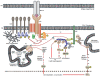
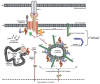
Nef, an accessory protein of the Human Immunodeficiency Virus type 1 (HIV-1), is dispensable for viral replication in cell culture, but promotes virus replication and pathogenesis in the infected host. Acting as protein-interaction adaptor, HIV-1 Nef modulates numerous target cell activities including cell surface receptor expression, cytoskeletal remodeling, vesicular transport, and signal transduction. In infected T-lymphocytes, altering T-cell antigen receptor (TCR) signaling has long been recognized as one key function of the viral protein. However, reported effects of Nef range from inhibition to activation of this cascade. Recent advances in the field begin to explain these seemingly contradictory observations and suggest that Nef alters intracellular trafficking of TCR proximal machinery to disrupt plasma membrane bound TCR signaling while at the same time, the viral protein induces localized signal transduction at the trans-Golgi network. This review summarizes these new findings on how HIV-1 Nef reprograms TCR signalling output from a broad response to selective activation of the RAS-Erk pathway. We also discuss the implications of these alterations in the context of HIV-1 infection and in light of current concepts of TCR signal transduction.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

