Lack of unique neuropathology in amyotrophic lateral sclerosis associated with p.K54E angiogenin (ANG) mutation
- PMID: 23228179
- PMCID: PMC3770927
- DOI: 10.1111/nan.12007
Lack of unique neuropathology in amyotrophic lateral sclerosis associated with p.K54E angiogenin (ANG) mutation
Abstract
Aims: Five to 10% of cases of amyotrophic lateral sclerosis are familial, with the most common genetic causes being mutations in the C9ORF72, SOD1, TARDBP and FUS genes. Mutations in the angiogenin gene, ANG, have been identified in both familial and sporadic patients in several populations within Europe and North America. The aim of this study was to establish the incidence of ANG mutations in a large cohort of 517 patients from Northern England and establish the neuropathology associated with these cases.
Methods: The single exon ANG gene was amplified, sequenced and analysed for mutations. Pathological examination of brain, spinal cord and skeletal muscle included conventional histology and immunohistochemistry.
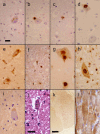
Results: Mutation screening identified a single sporadic amyotrophic lateral sclerosis case with a p.K54E mutation, which is absent from 278 neurologically normal control samples. The clinical presentation was of limb onset amyotrophic lateral sclerosis, with rapid disease progression and no evidence of cognitive impairment. Neuropathological examination established the presence of characteristic ubiquitinated and TDP-43-positive neuronal and glial inclusions, but no abnormality in the distribution of angiogenin protein.
Discussion: There is only one previous report describing the neuropathology in a single case with a p.K17I ANG mutation which highlighted the presence of eosinophilic neuronal intranuclear inclusions in the hippocampus. The absence of this feature in the present case indicates that patients with ANG mutations do not always have pathological changes distinguishable from those of sporadic amyotrophic lateral sclerosis.
Keywords: amyotrophic lateral sclerosis; angiogenin; glial inclusions; intranuclear inclusions; neuronal inclusions; neuropathology.
© 2012 The Authors. Neuropathology and Applied Neurobiology © 2012 British Neuropathological Society.
Figures


References
-
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. - PMC - PubMed
-
- Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. - PubMed
-
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair I, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. - PMC - PubMed
-
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

