[Anatomical heterogeneity in the proteome of human subcutaneous adipose tissue]
- PMID: 23228439
- PMCID: PMC4317363
- DOI: 10.1016/j.anpedi.2012.10.010
[Anatomical heterogeneity in the proteome of human subcutaneous adipose tissue]
Abstract
Background: Human subcutaneous (SQ) white adipose tissue (WAT) can vary according to its anatomical location, with subsequent differences in its proteomic profile.
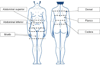
Patients and methods: SQ-WAT aspirates were obtained from six overweight (BMI>25kg/m(2)) women who underwent extensive liposuction. SQ-WAT was removed from six different locations (upper abdominal, lower abdominal, thigh, back, flank, and hip), and the protein profiles were determined by two-dimensional gel electrophoresis. In addition, the proteomic profiles of upper abdominal and hip SQ-WAT were subjected to further analysis, comparing samples obtained from two layers of WAT (deep and superficial).
Results: Twenty one protein spots showed differential intensities among the six defined anatomical locations, and 14 between the superficial and the deep layer. Among the proteins identified were, vimentin (structural protein), heat-shock proteins (HSPs), superoxide-dismutase (stress-resistance/chaperones), fatty-acid-binding protein (FABP) 4, and alpha-enolase (lipid and carbohydrate metabolism), and ATP-synthase (energy production). Among the WAT samples analyzed, the back sub-depot showed significant differences in the levels of selected proteins when compared to the other locations, with lower level of expression of several proteins involved in energy production and metabolism (ATP-synthase, alpha-enolase, HSPs and FABP-4).
Conclusions: The levels of several proteins in human SQ-WAT are not homogeneous between different WAT depots. These changes suggest the existence of inherent functional differences in subcutaneous fat depending upon its anatomical location. Thus, caution must be used when extrapolating data from one subcutaneous WAT region to other depots.
Introducción: El tejido adiposo blanco (TAB) subcutáneo (Sc) humano podría variar dependiendo de su localización anatómica, con diferencias en su perfil proteómico.
Pacientes y métodos: Se obtuvieron aspirados de TAB-Sc de seis mujeres con IMC >25 kg/m2, sometidas a liposucción. Dicho TAB-Sc se obtuvo de seis localizaciones anatómicas: abdominal superior e inferior, muslo, dorsal, flanco y cadera, analizándose su perfil proteómico mediante electroforesis bidimensional. En muslo y abdomen superior se compararon, además, las muestras obtenidas de las dos capas del TAB-Sc (profunda y superficial).
Resultados: Se detectaron 21 proteínas que mostraban una intensidad de expresión diferente entre las seis localizaciones anatómicas y 14 entre las capas superficial y profunda de una misma región. Entre las proteínas identificadas se incluyen: vimentina (proteína estructural); proteínas “heat-shock” (HSPs), superóxido-dismutasa, (estrés/chaperoninas); proteína fijadora de ácidos grasos 4 (FABP-4) y alfa-enolasa (metabolismo lipídico y de los hidratos de carbono, respectivamente) y ATP-sintetasa (producción de energía). Entre las regiones estudiadas, el TAB-Sc dorsal mostraba un perfil proteómico particular, con menor expresión de proteínas implicadas en la producción de energía y metabolismo (ATP-sintetasa, alfa-enolasa, HSPs y FABP-4) que el resto de regiones.
Conclusiones: Los niveles de expresión de diversas proteínas en el TAB-Sc humano no son homogéneos, difiriendo entre localizaciones anatómicas. Esto sugiere la existencia de diferencias funcionales en el TAB-Sc de acuerdo con su localización anatómica, lo que debe considerarse antes de asumir la extrapolación de los datos derivados del TAB-Sc de una determinada localización al de otras partes de la anatomía.
Copyright © 2012 Asociación Española de Pediatría. Published by Elsevier Espana. All rights reserved.
Figures



Similar articles
-
Heterogeneity among white adipose tissue depots in male C57BL/6J mice.Obesity (Silver Spring). 2012 Jan;20(1):101-11. doi: 10.1038/oby.2011.235. Epub 2011 Jul 21. Obesity (Silver Spring). 2012. PMID: 21779095 Free PMC article.
-
Proteome analysis of human adipocytes identifies depot-specific heterogeneity at metabolic control points.Am J Physiol Endocrinol Metab. 2021 Jun 1;320(6):E1068-E1084. doi: 10.1152/ajpendo.00473.2020. Epub 2021 Apr 12. Am J Physiol Endocrinol Metab. 2021. PMID: 33843278
-
Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice.Age (Dordr). 2012 Oct;34(5):1225-37. doi: 10.1007/s11357-011-9304-7. Epub 2011 Sep 29. Age (Dordr). 2012. PMID: 21953241 Free PMC article.
-
Adipose-derived stromal/stem cells from different adipose depots in obesity development.World J Stem Cells. 2019 Mar 26;11(3):147-166. doi: 10.4252/wjsc.v11.i3.147. World J Stem Cells. 2019. PMID: 30949294 Free PMC article. Review.
-
The origin and purpose of layers of subcutaneous adipose tissue in pigs and man.Horm Mol Biol Clin Investig. 2018 Mar 16;33(1). doi: 10.1515/hmbci-2018-0001. Horm Mol Biol Clin Investig. 2018. PMID: 29547390 Review.
Cited by
-
A proteomic approach to obesity and type 2 diabetes.J Cell Mol Med. 2015 Jul;19(7):1455-70. doi: 10.1111/jcmm.12600. Epub 2015 May 9. J Cell Mol Med. 2015. PMID: 25960181 Free PMC article. Review.
-
Identifying Key Genes and Functionally Enriched Pathways of Diverse Adipose Tissue Types in Cattle.Front Genet. 2022 Feb 14;13:790690. doi: 10.3389/fgene.2022.790690. eCollection 2022. Front Genet. 2022. PMID: 35237299 Free PMC article.
-
Obesity Proteomics: An Update on the Strategies and Tools Employed in the Study of Human Obesity.High Throughput. 2018 Sep 12;7(3):27. doi: 10.3390/ht7030027. High Throughput. 2018. PMID: 30213114 Free PMC article. Review.
-
The impact of growth hormone on proteomic profiles: a review of mouse and adult human studies.Clin Proteomics. 2017 Jun 29;14:24. doi: 10.1186/s12014-017-9160-2. eCollection 2017. Clin Proteomics. 2017. PMID: 28670222 Free PMC article. Review.
-
Proteomic analysis allows for early detection of potential markers of metabolic impairment in very young obese children.Int J Pediatr Endocrinol. 2014;2014(1):9. doi: 10.1186/1687-9856-2014-9. Epub 2014 Jun 10. Int J Pediatr Endocrinol. 2014. PMID: 24949022 Free PMC article.
References
-
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. - PubMed
-
- Enevoldsen LH, Simonsen L, Stallknecht B, Galbo H, Bulow J. In vivo human lipolytic activity in preperitoneal and subdivisions of subcutaneous abdominal adipose tissue. Am J Physiol Endocrinol Metab. 2001;281:E1110–E1114. - PubMed
-
- Tsuchida A, Yamauchi T, Kadowaki T. Nuclear receptors as targets for drug development: molecular mechanisms for regulation of obesity and insulin resistance by peroxisome proliferator-activated receptor gamma, CREB-binding protein, and adiponectin. J Pharmacol Sci. 2005;97:164–170. - PubMed
-
- Peinado JR, Jiménez-Gómez Y, Pulido MR, Ortega-Bellido M, Díaz-López C, Padillo FJ, et al. The stromal-vascular fraction of adipose tissue contributes to major differences between subcutaneous and visceral fat depots. Proteomics. 2010;10:3356–3366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials