A naonoporous cell-therapy device with controllable biodegradation for long-term drug release
- PMID: 23228849
- PMCID: PMC3777753
- DOI: 10.1016/j.jconrel.2012.11.020
A naonoporous cell-therapy device with controllable biodegradation for long-term drug release
Abstract
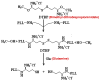
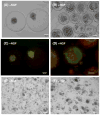
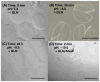
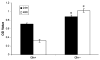
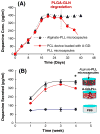
Herein we describe the development and implementation of a nanoporous cell-therapy device with controllable biodegradation. Dopamine-secreting PC12 cells were housed within newly formulated alginate-glutamine degradable polylysine (A-GD-PLL) microcapsules. The A-GD-PLL microcapsules provided a 3-D microenvironment for good spatial cell growth, viability and proliferation. The microcapsules were subsequently placed within a poly(ethylene glycol) (PEG)-coated poly(ε-caprolactone) (PCL) chamber covered with a PEG-grafted PCL nanoporous membrane formed by phase inversion. To enhance PC12 cell growth and to assist in controlled degradation of both the PC12 cells and the device construct, small PCL capsules containing neural growth factor (PCL-NGF) and a poly(lactic-co-glycolic acid) pellet containing glutamine (PLGA-GLN) were also placed within the PCL chamber. Release of NGF from the PCL-NGF capsules facilitated cell proliferation and viability, while the controlled release of GLN from the PLGA-GLN pellet resulted in A-GD-PLL microcapsule degradation and eventual PC12 cell death following a pre-specified period of time (4 weeks in this study). In vivo, our device was found to be well tolerated and we successfully demonstrated the controlled release of dopamine over a period of four weeks. This integrated biodegradable device holds great promise for the future treatment of a variety of diseases.
Published by Elsevier B.V.
Figures



References
-
- Wang NX, von Recum HA. Affinity-based drug delivery. Macromol Biosci. 2011;11:321–332. - PubMed
-
- Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006.
-
- Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17:1553–1561. - PubMed
-
- Gregoiraidis G. Engineered liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13:527–537. - PubMed
-
- Tao SL, Lubeley MW, Desai TA. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. J Control Release. 2003;88:215–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

