Antitubercular pharmacodynamics of phenothiazines
- PMID: 23228936
- PMCID: PMC3594496
- DOI: 10.1093/jac/dks483
Antitubercular pharmacodynamics of phenothiazines
Abstract
Objectives: Phenothiazines have been shown to exhibit in vitro and in vivo activity against Mycobacterium tuberculosis (Mtb) and multidrug-resistant Mtb. They are predicted to target the genetically validated respiratory chain component type II NADH:quinone oxidoreductase (Ndh). Using a set of compounds containing the phenothiazine pharmacophore, we have (i) investigated whether chemical validation data support the molecular target and (ii) evaluated pharmacophore tractability for further drug development.
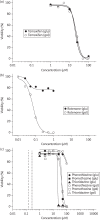
Methods: Recombinant Mtb Ndh was generated and its functionality confirmed by steady-state kinetics. Pharmacodynamic profiling of the phenothiazines, including antitubercular efficacy in aerobic and O2-limited conditions, time-kill assays and isobole analyses against first-line antituberculars, was performed. Potential mitochondrial toxicity was assessed in a modified HepG2 cell-line assay and against bovine cytochrome bc1.
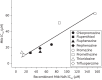
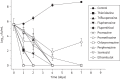
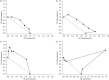
Results: Steady-state kinetic analyses revealed a substrate preference for coenzyme Q2 and an inability to utilize NADPH. A positive correlation between recombinant Ndh inhibition and kill of aerobically cultured Mtb was observed, whilst enhanced potency was demonstrated in a hypoxic model. Time-kill studies revealed the phenothiazines to be bactericidal whilst isobolograms exposed antagonism with isoniazid, indicative of intracellular NADH/NAD(+) couple perturbation. At therapeutic levels, phenothiazine-mediated toxicity was appreciable; however, specific mitochondrial targeting was excluded.
Conclusions: Data generated support the hypothesis that Ndh is the molecular target of phenothiazines. The favourable pharmacodynamic properties of the phenothiazines are consistent with a target product profile that includes activity against dormant/persistent bacilli, rapid bactericidal activity and activity against drug-resistant Mtb by a previously unexploited mode of action. These properties warrant further medicinal chemistry to improve potency and safety.
Figures


Similar articles
-
Steady-state kinetics and inhibitory action of antitubercular phenothiazines on mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2).J Biol Chem. 2006 Apr 28;281(17):11456-63. doi: 10.1074/jbc.M508844200. Epub 2006 Feb 9. J Biol Chem. 2006. PMID: 16469750
-
Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs.Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4548-53. doi: 10.1073/pnas.0500469102. Epub 2005 Mar 14. Proc Natl Acad Sci U S A. 2005. PMID: 15767566 Free PMC article.
-
Type-II NADH Dehydrogenase (NDH-2): a promising therapeutic target for antitubercular and antibacterial drug discovery.Expert Opin Ther Targets. 2017 Jun;21(6):559-570. doi: 10.1080/14728222.2017.1327577. Epub 2017 May 15. Expert Opin Ther Targets. 2017. PMID: 28472892 Review.
-
Antitubercular polyhalogenated phenothiazines and phenoselenazine with reduced binding to CNS receptors.Eur J Med Chem. 2020 Sep 1;201:112420. doi: 10.1016/j.ejmech.2020.112420. Epub 2020 Jun 5. Eur J Med Chem. 2020. PMID: 32526553
-
Type II NADH: menaquinone oxidoreductase of Mycobacterium tuberculosis.Infect Disord Drug Targets. 2007 Jun;7(2):169-81. doi: 10.2174/187152607781001781. Infect Disord Drug Targets. 2007. PMID: 17970227 Review.
Cited by
-
Towards Property Profiling: SYNTHESIS and SAR Probing of New Tetracyclic Diazaphenothiazine Analogues.Int J Mol Sci. 2021 Nov 26;22(23):12826. doi: 10.3390/ijms222312826. Int J Mol Sci. 2021. PMID: 34884631 Free PMC article.
-
Characterization of the type 2 NADH:menaquinone oxidoreductases from Staphylococcus aureus and the bactericidal action of phenothiazines.Biochim Biophys Acta. 2014 Jul;1837(7):954-63. doi: 10.1016/j.bbabio.2014.03.017. Epub 2014 Apr 5. Biochim Biophys Acta. 2014. PMID: 24709059 Free PMC article.
-
Antimalarial 4(1H)-pyridones bind to the Qi site of cytochrome bc1.Proc Natl Acad Sci U S A. 2015 Jan 20;112(3):755-60. doi: 10.1073/pnas.1416611112. Epub 2015 Jan 6. Proc Natl Acad Sci U S A. 2015. PMID: 25564664 Free PMC article.
-
Identification of 2-Aryl-Quinolone Inhibitors of Cytochrome bd and Chemical Validation of Combination Strategies for Respiratory Inhibitors against Mycobacterium tuberculosis.ACS Infect Dis. 2023 Feb 10;9(2):221-238. doi: 10.1021/acsinfecdis.2c00283. Epub 2023 Jan 6. ACS Infect Dis. 2023. PMID: 36606559 Free PMC article.
-
Design, Synthesis and Antimicrobial Properties of New Tetracyclic Quinobenzothiazine Derivatives.Int J Mol Sci. 2022 Dec 1;23(23):15078. doi: 10.3390/ijms232315078. Int J Mol Sci. 2022. PMID: 36499402 Free PMC article.
References
-
- WHO. Global Tuberculosis Control 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. (17 September 2012, date last accessed)
-
- Ginsberg AM. Drugs in development for tuberculosis. Drugs. 2010;70:2201–14. - PubMed
-
- Cole ST, Riccardi G. New tuberculosis drugs on the horizon. Curr Opin Microbiol. 2011;14:570–6. - PubMed
-
- Koul A, Dendouga N, Vergauwen K, et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol. 2007;3:323–4. - PubMed
-
- Koul A, Vranckx L, Dendouga N, et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem. 2008;283:25273–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

