The CshA DEAD-box RNA helicase is important for quorum sensing control in Staphylococcus aureus
- PMID: 23229022
- PMCID: PMC3590232
- DOI: 10.4161/rna.22899
The CshA DEAD-box RNA helicase is important for quorum sensing control in Staphylococcus aureus
Abstract
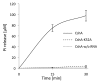
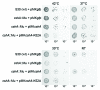
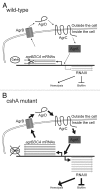
DEAD-box RNA helicases are present in almost all living organisms and participate in various processes of RNA metabolism. Bacterial proteins of this large family were shown to be required for translation initiation, ribosome biogenesis and RNA decay. The latter is primordial for rapid adaptation to changing environmental conditions. In particular, the RhlB RNA helicase from E. coli was shown to assist the bacterial degradosome machinery. Recently, the CshA DEAD-box proteins from Bacillus subtilis and Staphylococcus aureus were shown to interact with proteins that are believed to form the degradosome. S. aureus can cause life-threatening disease, with particular concern focusing on biofilm formation on catheters and prosthetic devices, since in this form the bacteria are almost impossible to eradicate both by the immune system and antibiotic treatment. This persistent state relies on the expression of surface encoded proteins that allow attachment to various surfaces, and contrasts with the dispersal mode of growth that relies on the secretion of proteins such as hemolysins and proteases. The switch between these two states is mainly mediated by the Staphylococcal cell density sensing system encoded by agr. We show that inactivation of the cshA DEAD-box gene results in dysregulation of biofilm formation and hemolysis through modulation of agr mRNA stability. Importantly, inactivation of the agrA gene in the cshA mutant background reverses the defect, indicating that cshA is genetically upstream of agr and that a delicate balance of agr mRNA abundance mediated through stability control by CshA is critical for proper expression of virulence factors.
Keywords: RNA helicase; Staphylococcus aureus; degradosome; quorum sensing.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
