Ischaemia-reperfusion injury in liver transplantation--from bench to bedside
- PMID: 23229329
- PMCID: PMC3577927
- DOI: 10.1038/nrgastro.2012.225
Ischaemia-reperfusion injury in liver transplantation--from bench to bedside
Abstract
Ischaemia-reperfusion injury (IRI) in the liver, a major complication of haemorrhagic shock, resection and transplantation, is a dynamic process that involves the two interrelated phases of local ischaemic insult and inflammation-mediated reperfusion injury. This Review highlights the latest mechanistic insights into innate-adaptive immune crosstalk and cell activation cascades that lead to inflammation-mediated injury in livers stressed by ischaemia-reperfusion, discusses progress in large animal experiments and examines efforts to minimize liver IRI in patients who have received a liver transplant. The interlinked signalling pathways in multiple hepatic cell types, the IRI kinetics and positive versus negative regulatory loops at the innate-adaptive immune interface are discussed. The current gaps in our knowledge and the pathophysiology aspects of IRI in which basic and translational research is still required are stressed. An improved appreciation of cellular immune events that trigger and sustain local inflammatory responses, which are ultimately responsible for organ injury, is fundamental to developing innovative strategies for treating patients who have received a liver transplant and developed ischaemia-reperfusion inflammation and organ dysfunction.
Conflict of interest statement
The authors declare no competing interests.
Figures


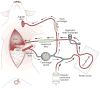
References
-
- US Department of Health and Human Services. Organ Procurement and Transplantation Network. 2012 [online], http://optn.transplant.hrsa.gov/data/
-
- Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

