The experimental evaluation and molecular dynamics simulation of a heat-enhanced transdermal delivery system
- PMID: 23229382
- PMCID: PMC3581683
- DOI: 10.1208/s12249-012-9900-6
The experimental evaluation and molecular dynamics simulation of a heat-enhanced transdermal delivery system
Abstract
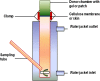
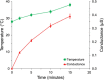
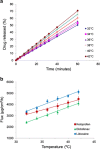
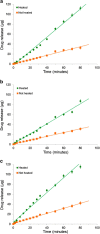
Transdermal delivery systems are useful in cases where preferred routes such as the oral route are not available. However, low overall extent of delivery is seen due to the permeation barrier posed by the skin. Chemical penetration enhancers and invasive methods that disturb the structural barrier function of the skin can be used to improve transdermal drug delivery. However, for suitable drugs, a fast-releasing transdermal delivery system can be produced by incorporating a heating source into a transdermal patch. In this study, a molecular dynamics simulation showed that heat increased the diffusivity of the drug molecules, resulting in faster release from gels containing ketoprofen, diclofenac sodium, and lidocaine HCl. Simulations were confirmed by in vitro drug release studies through lipophilic membranes. These correlations could expand the application of heated transdermal delivery systems for use as fast-release-dosage forms.
Figures




Similar articles
-
Evaluation of Heat Effects on Fentanyl Transdermal Delivery Systems Using In Vitro Permeation and In Vitro Release Methods.J Pharm Sci. 2020 Oct;109(10):3095-3104. doi: 10.1016/j.xphs.2020.07.013. Epub 2020 Jul 20. J Pharm Sci. 2020. PMID: 32702372 Free PMC article.
-
Effect of Mild Hyperthermia on Transdermal Absorption of Nicotine from Patches.AAPS PharmSciTech. 2019 Jan 11;20(2):77. doi: 10.1208/s12249-019-1299-x. AAPS PharmSciTech. 2019. PMID: 30635802
-
Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery.Curr Med Chem. 2000 Jun;7(6):593-608. doi: 10.2174/0929867003374840. Curr Med Chem. 2000. PMID: 10702628 Review.
-
Enhanced transdermal delivery of ketoprofen from bioadhesive gels.Pak J Pharm Sci. 2009 Apr;22(2):193-8. Pak J Pharm Sci. 2009. PMID: 19339232
-
Transdermal delivery of contraceptives.Crit Rev Ther Drug Carrier Syst. 1990;7(2):149-86. Crit Rev Ther Drug Carrier Syst. 1990. PMID: 2272099 Review.
Cited by
-
Evaluation of physical and chemical modifications to drug reservoirs for stimuli-responsive microneedles.Drug Deliv Transl Res. 2025 Jul;15(7):2390-2414. doi: 10.1007/s13346-024-01737-0. Epub 2024 Nov 20. Drug Deliv Transl Res. 2025. PMID: 39565514 Free PMC article.
-
Heat effects on drug delivery across human skin.Expert Opin Drug Deliv. 2016;13(5):755-68. doi: 10.1517/17425247.2016.1136286. Epub 2016 Jan 25. Expert Opin Drug Deliv. 2016. PMID: 26808472 Free PMC article. Review.
-
A Molecular Interpretation on the Different Penetration Enhancement Effect of Borneol and Menthol towards 5-Fluorouracil.Int J Mol Sci. 2017 Dec 18;18(12):2747. doi: 10.3390/ijms18122747. Int J Mol Sci. 2017. PMID: 29258240 Free PMC article.
-
Modulating phase segregation during spin-casting of fullerene-based polymer solar-cell thin films upon minor addition of a high-boiling co-solvent.J Appl Crystallogr. 2024 Nov 17;57(Pt 6):1871-1883. doi: 10.1107/S1600576724010082. eCollection 2024 Dec 1. J Appl Crystallogr. 2024. PMID: 39628884 Free PMC article.
-
Mechanistic Insights Underlying the Drug Release and Skin Permeation of Guanfacine Transdermal Patch with Various Acrylic Pressure-Sensitive Adhesives.AAPS PharmSciTech. 2025 Jan 8;26(1):26. doi: 10.1208/s12249-024-03031-1. AAPS PharmSciTech. 2025. PMID: 39779644
References
-
- Muktadir A, Barbar A, Cutie AJ, Plakogiannis FM. Medicament release from ointment bases. III. Ibuprofen: in vitro release and in-vivo absorption in rabbits. Drug Dev Ind Pharm. 1986;12:2521–2540. doi: 10.3109/03639048609063197. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

