Understanding how noncatalytic carbohydrate binding modules can display specificity for xyloglucan
- PMID: 23229556
- PMCID: PMC3576085
- DOI: 10.1074/jbc.M112.432781
Understanding how noncatalytic carbohydrate binding modules can display specificity for xyloglucan
Abstract
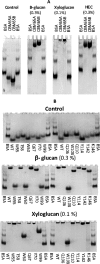
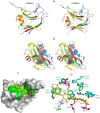
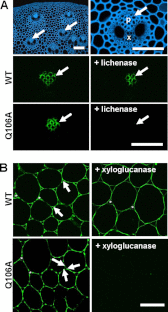
Plant biomass is central to the carbon cycle and to environmentally sustainable industries exemplified by the biofuel sector. Plant cell wall degrading enzymes generally contain noncatalytic carbohydrate binding modules (CBMs) that fulfil a targeting function, which enhances catalysis. CBMs that bind β-glucan chains often display broad specificity recognizing β1,4-glucans (cellulose), β1,3-β1,4-mixed linked glucans and xyloglucan, a β1,4-glucan decorated with α1,6-xylose residues, by targeting structures common to the three polysaccharides. Thus, CBMs that recognize xyloglucan target the β1,4-glucan backbone and only accommodate the xylose decorations. Here we show that two closely related CBMs, CBM65A and CBM65B, derived from EcCel5A, a Eubacterium cellulosolvens endoglucanase, bind to a range of β-glucans but, uniquely, display significant preference for xyloglucan. The structures of the two CBMs reveal a β-sandwich fold. The ligand binding site comprises the β-sheet that forms the concave surface of the proteins. Binding to the backbone chains of β-glucans is mediated primarily by five aromatic residues that also make hydrophobic interactions with the xylose side chains of xyloglucan, conferring the distinctive specificity of the CBMs for the decorated polysaccharide. Significantly, and in contrast to other CBMs that recognize β-glucans, CBM65A utilizes different polar residues to bind cellulose and mixed linked glucans. Thus, Gln(106) is central to cellulose recognition, but is not required for binding to mixed linked glucans. This report reveals the mechanism by which β-glucan-specific CBMs can distinguish between linear and mixed linked glucans, and show how these CBMs can exploit an extensive hydrophobic platform to target the side chains of decorated β-glucans.
Figures


References
-
- Flint H. J., Duncan S. H., Scott K. P., Louis P. (2007) Interactions and competition within the microbial community of the human colon. Links between diet and health. Environ. Microbiol. 9, 1101–1111 - PubMed
-
- Mackie R. I., White B. A. (1990) Recent advances in rumen microbial ecology and metabolism. Potential impact on nutrient output. J. Dairy Sci. 73, 2971–2995 - PubMed
-
- Himmel M. E., Bayer E. A. (2009) Lignocellulose conversion to biofuels. Current challenges, global perspectives. Curr. Opin. Biotechnol. 20, 316–317 - PubMed
-
- Himmel M. E., Ding S. Y., Johnson D. K., Adney W. S., Nimlos M. R., Brady J. W., Foust T. D. (2007) Biomass recalcitrance. Engineering plants and enzymes for biofuels production. Science 315, 804–807 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

