Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice
- PMID: 23230000
- PMCID: PMC3526355
- DOI: 10.1084/jem.20121015
Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice
Abstract
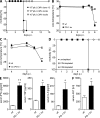
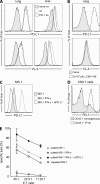
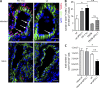
The inhibitory programmed death 1 (PD-1)-programmed death ligand 1 (PD-L1) pathway contributes to the functional down-regulation of T cell responses during persistent systemic and local virus infections. The blockade of PD-1-PD-L1-mediated inhibition is considered as a therapeutic approach to reinvigorate antiviral T cell responses. Yet previous studies reported that PD-L1-deficient mice develop fatal pathology during early systemic lymphocytic choriomeningitis virus (LCMV) infection, suggesting a host protective role of T cell down-regulation. As the exact mechanisms of pathology development remained unclear, we set out to delineate in detail the underlying pathogenesis. Mice deficient in PD-1-PD-L1 signaling or lacking PD-1 signaling in CD8 T cells succumbed to fatal CD8 T cell-mediated immunopathology early after systemic LCMV infection. In the absence of regulation via PD-1, CD8 T cells killed infected vascular endothelial cells via perforin-mediated cytolysis, thereby severely compromising vascular integrity. This resulted in systemic vascular leakage and a consequential collapse of the circulatory system. Our results indicate that the PD-1-PD-L1 pathway protects the vascular system from severe CD8 T cell-mediated damage during early systemic LCMV infection, highlighting a pivotal physiological role of T cell down-regulation and suggesting the potential development of immunopathological side effects when interfering with the PD-1-PD-L1 pathway during systemic virus infections.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

