Polymerase ε1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature ("FILS syndrome")
- PMID: 23230001
- PMCID: PMC3526359
- DOI: 10.1084/jem.20121303
Polymerase ε1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature ("FILS syndrome")
Abstract
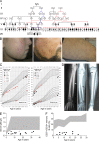
DNA polymerase ε (Polε) is a large, four-subunit polymerase that is conserved throughout the eukaryotes. Its primary function is to synthesize DNA at the leading strand during replication. It is also involved in a wide variety of fundamental cellular processes, including cell cycle progression and DNA repair/recombination. Here, we report that a homozygous single base pair substitution in POLE1 (polymerase ε 1), encoding the catalytic subunit of Polε, caused facial dysmorphism, immunodeficiency, livedo, and short stature ("FILS syndrome") in a large, consanguineous family. The mutation resulted in alternative splicing in the conserved region of intron 34, which strongly decreased protein expression of Polε1 and also to a lesser extent the Polε2 subunit. We observed impairment in proliferation and G1- to S-phase progression in patients' T lymphocytes. Polε1 depletion also impaired G1- to S-phase progression in B lymphocytes, chondrocytes, and osteoblasts. Our results evidence the developmental impact of a Polε catalytic subunit deficiency in humans and its causal relationship with a newly recognized, inherited disorder.
Figures


References
-
- Albertson T.M., Ogawa M., Bugni J.M., Hays L.E., Chen Y., Wang Y., Treuting P.M., Heddle J.A., Goldsby R.E., Preston B.D.. 2009. DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice. Proc. Natl. Acad. Sci. USA. 106:17101–17104. 10.1073/pnas.0907147106 - DOI - PMC - PubMed
-
- Braegger C., Jenni O., Konrad D., Molinari L.. 2011. Neue Wachstumskurven für die Schweiz. Paediatrica. 1:9–11.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

