Oseltamivir pharmacokinetics, dosing, and resistance among children aged <2 years with influenza
- PMID: 23230059
- PMCID: PMC3563309
- DOI: 10.1093/infdis/jis765
Oseltamivir pharmacokinetics, dosing, and resistance among children aged <2 years with influenza
Abstract
Background: Children <2 years of age are at high risk of influenza-related mortality and morbidity. However, the appropriate dose of oseltamivir for children <2 years of age is unknown.
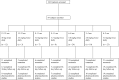
Methods: The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group evaluated oseltamivir in infants aged <2 years in an age-de-escalation, adaptive design with a targeted systemic exposure.
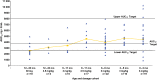
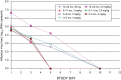
Results: From 2006 to 2010, 87 subjects enrolled. An oseltamivir dose of 3.0 mg/kg produced drug exposures within the target range in subjects 0-8 months of age, although there was a greater degree of variability in infants <3 months of age. In subjects 9-11 months of age, a dose of 3.5 mg/kg produced drug exposures within the target range. Six of 10 subjects aged 12-23 months receiving the Food and Drug Administration-approved unit dose for this age group (ie, 30 mg) had oseltamivir carboxylate exposures below the target range. Virus from 3 subjects developed oseltamivir resistance during antiviral treatment.
Conclusions: The appropriate twice-daily oral oseltamivir dose for infants ≤8 months of age is 3.0 mg/kg, while the dose for infants 9-11 months old is 3.5 mg/kg.
Trial registration: ClinicalTrials.gov NCT00391768.
Figures
Comment in
-
[Pharmacokinetics of oseltamivir in children younger than 2 years with influenza].Rev Chilena Infectol. 2013 Apr;30(2):223. doi: 10.4067/S0716-10182013000200015. Rev Chilena Infectol. 2013. PMID: 23677163 Spanish. No abstract available.
References
-
- Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003—2004. N. Engl. J. Med. 2005;353:2559–67. - PubMed
-
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N. Engl. J. Med. 2000;342:232–9. - PubMed
-
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 2000;342:225–31. - PubMed
-
- Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–64. - PubMed
-
- O'Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004;113:585–93. - PubMed

