New insights into the determination of HDL structure by apolipoproteins: Thematic review series: high density lipoprotein structure, function, and metabolism
- PMID: 23230082
- PMCID: PMC3708355
- DOI: 10.1194/jlr.R034025
New insights into the determination of HDL structure by apolipoproteins: Thematic review series: high density lipoprotein structure, function, and metabolism
Abstract
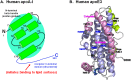
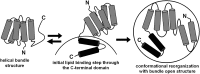
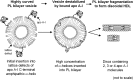
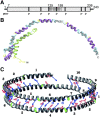
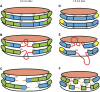
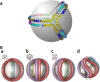
Apolipoprotein (apo)A-I is the principal protein component of HDL, and because of its conformational adaptability, it can stabilize all HDL subclasses. The amphipathic α-helix is the structural motif that enables apoA-I to achieve this functionality. In the lipid-free state, the helical segments unfold and refold in seconds and are located in the N-terminal two thirds of the molecule where they are loosely packed as a dynamic, four-helix bundle. The C-terminal third of the protein forms an intrinsically disordered domain that mediates initial binding to phospholipid surfaces, which occurs with coupled α-helix formation. The lipid affinity of apoA-I confers detergent-like properties; it can solubilize vesicular phospholipids to create discoidal HDL particles with diameters of approximately 10 nm. Such particles contain a segment of phospholipid bilayer and are stabilized by two apoA-I molecules that are arranged in an anti-parallel, double-belt conformation around the edge of the disc, shielding the hydrophobic phospholipid acyl chains from exposure to water. The apoA-I molecules are in a highly dynamic state, and they stabilize discoidal particles of different sizes by certain segments forming loops that detach reversibly from the particle surface. The flexible apoA-I molecule adapts to the surface of spherical HDL particles by bending and forming a stabilizing trefoil scaffold structure. The above characteristics of apoA-I enable it to partner with ABCA1 in mediating efflux of cellular phospholipid and cholesterol and formation of a heterogeneous population of nascent HDL particles. Novel insights into the structure-function relationships of apoA-I should help reveal mechanisms by which HDL subclass distribution can be manipulated.
Keywords: ATP binding cassette transporter A1; amphipathic α-helix; apoA-I; apoE; cholesterol; helix bundle; lipoprotein; membrane solubilization; phospholipid.
Figures



References
-
- Lund-Katz S., Liu L., Thuahnai S. T., Phillips M. C. 2003. High density lipoprotein structure. Front. Biosci. 8: d1044–d1054 - PubMed
-
- Kontush A., Chapman M. J. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374 - PubMed
-
- Jonas A., Phillips M. C. 2008. Lipoprotein. In Biochemistry structure of Lipids, Lipoproteins and Membranes. 5th edition. D. E. Vance and J. E. Vance, editors. Elsevier, Oxford, UK. 485–506.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

