Modulation of oxidative stability of haemoglobin inside liposome-encapsulated haemoglobin
- PMID: 23231644
- PMCID: PMC3696053
- DOI: 10.3109/02652048.2012.752535
Modulation of oxidative stability of haemoglobin inside liposome-encapsulated haemoglobin
Abstract
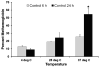
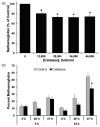
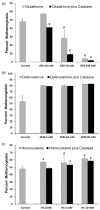
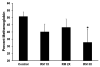
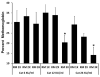
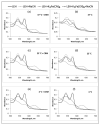
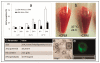
The major hurdle in the formulation of liposome-encapsulated haemoglobin (LEH) is the oxidation of haemoglobin (Hb) into methaemoglobin during storage and after administration. In order to reduce this oxidative degradation, we tested various reducing conditions in the presence of catalase. We found that at 37°C more than 50% of Hb oxidized to methaemoglobin within 24 h, whereas in presence of catalase, the oxidation was significantly reduced. The effect of catalase was further enhanced by a reduction mixture containing β-NAD, d-glucose, adenine, inosine, MgCl2, KCl, KH2PO4 and Na2HPO4; only 14% methaemoglobin was generated in the presence of catalase and reduction mixture. Contrary to the expectation, glutathione, deferoxamine and homocysteine enhanced Hb oxidation. The presence of CRM inside liposomes (250 nm) significantly decreased Hb oxidation. The results suggest that catalase and a well-defined mixture of co-factors may help control Hb oxidation for improvement in the functional life of LEH.
Figures
Similar articles
-
Preparation and characterization of liposome-encapsulated haemoglobin by a freeze-thaw method.J Microencapsul. 1994 Jul-Aug;11(4):409-21. doi: 10.3109/02652049409034258. J Microencapsul. 1994. PMID: 7931940
-
Simple method for preparing poly(ethylene glycol)-surface-conjugated liposome-encapsulated hemoglobins: physicochemical properties, long-term storage stability, and their reactions with O2, CO, and NO.Langmuir. 2011 Jul 19;27(14):8829-40. doi: 10.1021/la201246m. Epub 2011 Jun 16. Langmuir. 2011. PMID: 21678920 Free PMC article.
-
Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes.Biomaterials. 2005 Jun;26(17):3759-69. doi: 10.1016/j.biomaterials.2004.09.015. Biomaterials. 2005. PMID: 15621266
-
Haemoglobin-based blood substitutes: what's on the horizon?Ann Acad Med Singap. 1994 Nov;23(6 Suppl):71-6. Ann Acad Med Singap. 1994. PMID: 7710240 Review.
-
Approach to clinical trial considering medical ethics and efficacy for HbV, liposome encapsulated hemoglobin vesicle.Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):65-73. doi: 10.1081/bio-200046695. Artif Cells Blood Substit Immobil Biotechnol. 2005. PMID: 15768566 Review.
Cited by
-
Silk Fibroin Particles as Carriers in the Development of All-Natural Hemoglobin-Based Oxygen Carriers (HBOCs).bioRxiv [Preprint]. 2023 Mar 2:2023.03.01.530637. doi: 10.1101/2023.03.01.530637. bioRxiv. 2023. Update in: Adv Nanobiomed Res. 2023 Sep;3(9):2300019. doi: 10.1002/anbr.202300019. PMID: 36909572 Free PMC article. Updated. Preprint.
-
Imbalance of Homocysteine and H2S: Significance, Mechanisms, and Therapeutic Promise in Vascular Injury.Oxid Med Cell Longev. 2019 Nov 22;2019:7629673. doi: 10.1155/2019/7629673. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885816 Free PMC article. Review.
-
Silk Fibroin Particles as Carriers in the Development of Hemoglobin-Based Oxygen Carriers.Adv Nanobiomed Res. 2023 Sep;3(9):2300019. doi: 10.1002/anbr.202300019. Epub 2023 Jul 27. Adv Nanobiomed Res. 2023. PMID: 38708087 Free PMC article.
References
-
- Akintonwa DA. Theoretical mechanistic basis of oxidants of methaemoglobin formation. Med Hypotheses. 2000;54:312–20. - PubMed
-
- Alayash AI. Oxidative mechanisms of hemoglobin-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 2001;29:415–25. - PubMed
-
- Alayash AI, Ryan BA, Fratantoni JC, Cashon RE. Redox reactivity of modified hemoglobins with hydrogen peroxide and nitric oxide: Toxicological implications. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:373–86. - PubMed
-
- Arnoldo BD, Minei JP. Potential of hemoglobin-based oxygen carriers in trauma patients. Curr Opin Crit Care. 2001;7:431–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
