Highly efficient site-specific transgenesis in cancer cell lines
- PMID: 23231822
- PMCID: PMC3537676
- DOI: 10.1186/1476-4598-11-89
Highly efficient site-specific transgenesis in cancer cell lines
Abstract
Background: Transgenes introduced into cancer cell lines serve as powerful tools for identification of genes involved in cancer. However, the random nature of genomic integration site of a transgene highly influences the fidelity, reliability and level of its expression. In order to alleviate this bottleneck, we characterized the potential utility of a novel PhiC31 integrase-mediated site-specific insertion system (PhiC31-IMSI) for introduction of transgenes into a pre-inserted docking site in the genome of cancer cells.
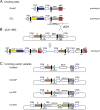
Methods: According to this system, a "docking-site" was first randomly inserted into human cancer cell lines and clones with a single copy were selected. Subsequently, an "incoming" vector containing the gene of interest was specifically inserted in the docking-site using PhiC31.
Results: Using the Pc-3 and SKOV-3 cancer cell lines, we showed that transgene insertion is reproducible and reliable. Furthermore, the selection system ensured that all surviving stable transgenic lines harbored the correct integration site. We demonstrated that the expression levels of reporter genes, such as green fluorescent protein and luciferase, from the same locus were comparable among sister, isogenic clones. Using in vivo xenograft studies, we showed that the genetically altered cancer cell lines retain the properties of the parental line. To achieve temporal control of transgene expression, we coupled our insertion strategy with the doxycycline inducible system and demonstrated tight regulation of the expression of the antiangiogenic molecule sFlt-1-Fc in Pc-3 cells. Furthermore, we introduced the luciferase gene into the insertion cassette allowing for possible live imaging of cancer cells in transplantation assays. We also generated a series of Gateway cloning-compatible intermediate cassettes ready for high-throughput cloning of transgenes and demonstrated that PhiC31-IMSI can be achieved in a high throughput 96-well plate format.
Conclusions: The novel PhiC31-IMSI system described in this study represents a powerful tool that can facilitate the characterization of cancer-related genes.
Figures
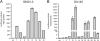

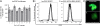

References
-
- Cimoli G, Malacarne D, Ponassi R, Valenti M, Alberti S, Parodi S. Meta-analysis of the role of p53 status in isogenic systems tested for sensitivity to cytotoxic antineoplastic drugs. Biochim Biophys Acta. 2004;1705(2):103–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

